Live vaccine, live vaccine freeze-dried powder, live vaccine protective agent as well as preparation method and application thereof
A technology of live vaccine and protective agent, applied in vaccines, freeze-dried delivery, veterinary vaccines, etc., can solve the problems of immunization failure, difficulty in storage, transportation and use, and failure to protect animals.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0035] The present invention provides a preparation method of the live vaccine protective agent described in the above technical scheme, comprising the following steps:
[0036] Mixing water-soluble gelatin, oligosaccharides, sorbitol, polyvinylpyrrolidone and the first remaining water for injection for high-temperature sterilization to obtain the first raw material;
[0037] After mixing and filtering L-sodium glutamate, sodium ascorbate, 199 medium and the second remaining water for injection, the second raw material is obtained;
[0038] After the first raw material is mixed with the second raw material, the insufficient part is made up by the third water for injection to obtain a live vaccine protective agent.
[0039] The present invention mixes water-soluble gelatin, oligosaccharide, sorbitol, polyvinylpyrrolidone K30 and the first remaining water for injection for high-temperature sterilization to obtain the first raw material. In the present invention, the amount of t...
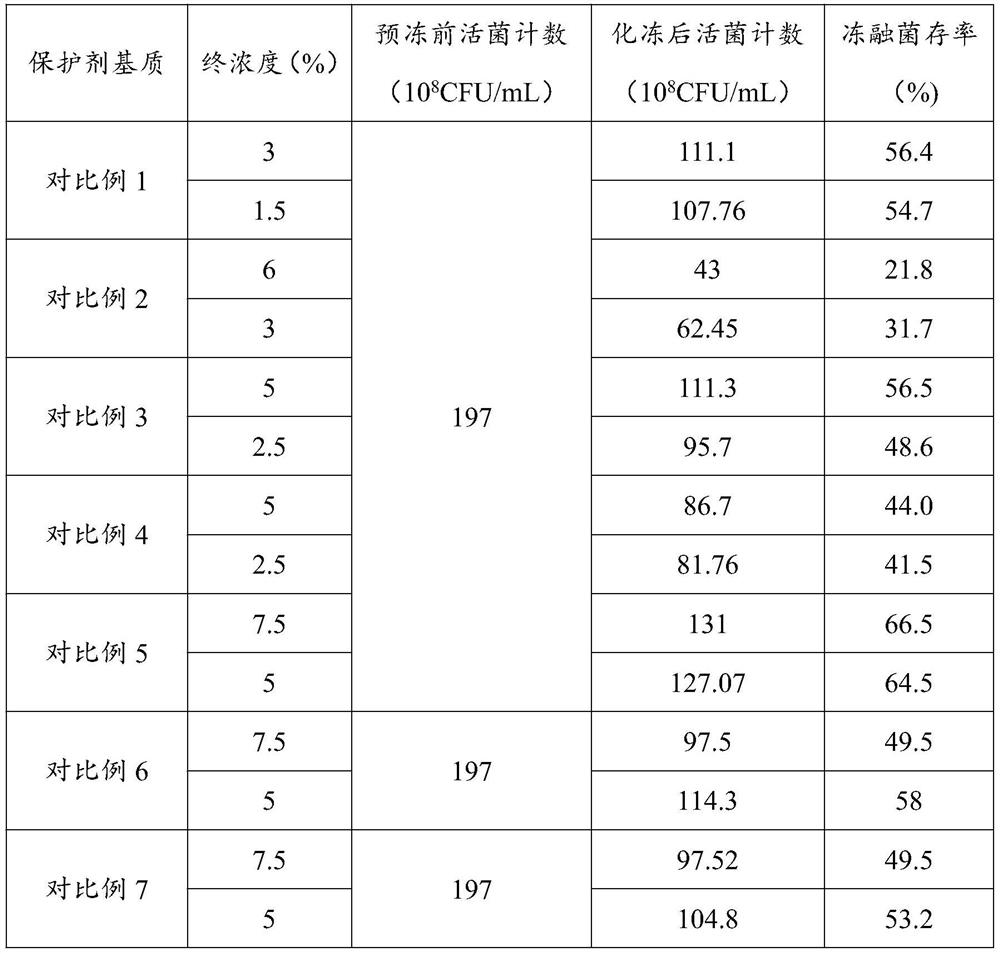
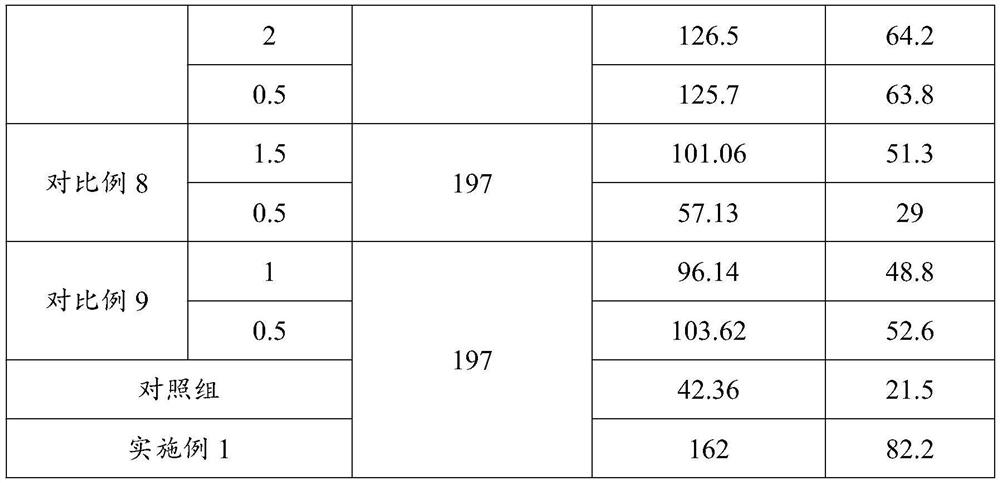
Embodiment 1
[0061] Raw material preparation: 1.5g water-soluble gelatin, 5g sucrose, 5g sorbitol, 0.1g polyvinylpyrrolidone K30, 5g L-sodium glutamate, 1.5g sodium ascorbate, 0.5g 199 culture medium, and make up the balance to 100mL with distilled water .
[0062] Mix 1.5g of water-soluble gelatin, 5g of sucrose, 5g of sorbitol, 0.1g of polyvinylpyrrolidone K30 and 30mL of water for injection, and sterilize at 25°C for 25 minutes to obtain the first raw material;
[0063] After mixing and filtering 5 g of L-sodium glutamate, 1.5 g of sodium ascorbate, 0.5 g of 199 medium and 30 mL of water for injection, the second raw material was obtained;
[0064]After mixing the first raw material and the second raw material, the insufficient part is made up with sterilized water for injection to obtain the live vaccine protective agent.
Embodiment 2
[0066] Preparation of Bacterial Solution for Yak Paratyphoid Live Vaccine
[0067] The seed solution was prepared according to the requirements of "Regulations of Veterinary Biological Products of the People's Republic of China" (2000 edition, Ministry of Agriculture of the People's Republic of China.-Beijing: Chemical Industry Press, 2000, hereinafter referred to as "Regulations").
[0068] Inoculate the seed solution at 2% into 5 bottles of sterilized 200mL nutrient broth medium, place it at 37°C for 185r / min to ferment and cultivate for 17h, and centrifuge the bacterial solution at 3500r / min for 20min to collect all the sludge and use Suspended in sterilized physiological saline, mixed thoroughly, and used as a bacterial solution for seedling production.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com