5-boron pyridine carboxylic acid modified targeted drug delivery system and preparation method thereof
A boracic acid delivery system technology, applied in drug delivery, pharmaceutical formulation, drug combination, etc., can solve the problems of limited boric acid derivatives, improve biocompatibility and stability, improve drug delivery effect, good The effect of biocompatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
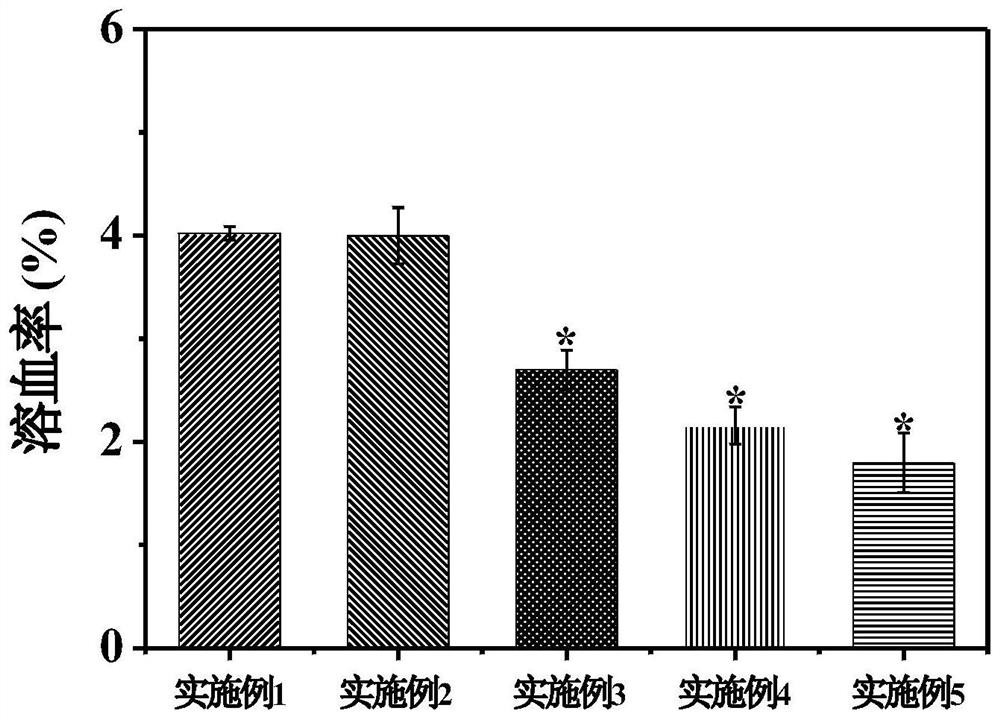
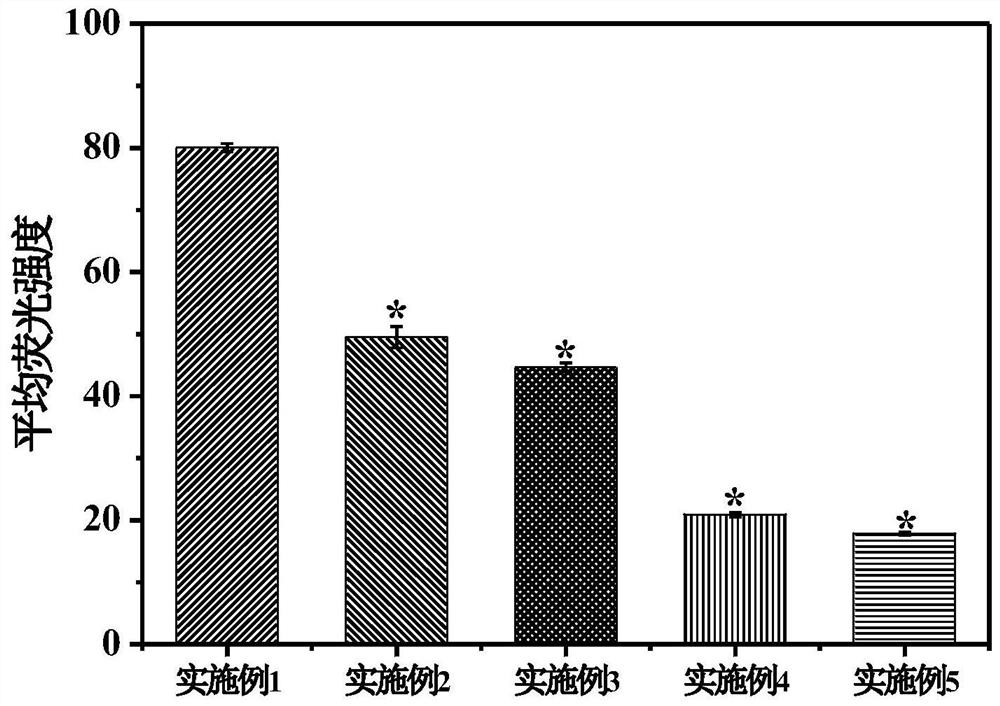
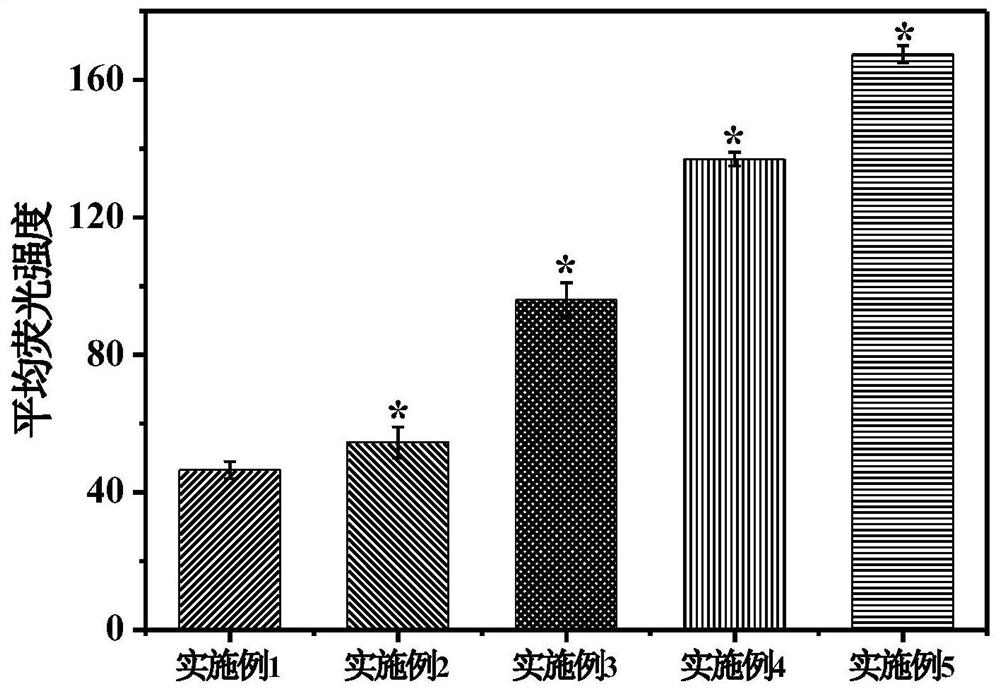
Embodiment 1
[0032] This example is a control example. In this example, no modification with 5-boropyridine carboxylic acid is used, and only drug-loaded nanoparticles are used alone. The preparation method of this embodiment is:
[0033] S1, using two monomers of lactide (LA) and glycolide (GA) for ring-opening copolymerization to obtain a polymer (PLGA), which is then functionalized and reacted with polyethyleneimine (PEI) for amidation reaction, thereby Graft PEI to prepare PLGA-PEI amphiphilic copolymer.
[0034] The specific steps of S1 are:
[0035] ① Using 1,8-octanediol as the initiator and stannous octoate as the catalyst, the PLGA copolymer was synthesized by ring-opening polymerization of LA and GA monomers;
[0036] 2. Esterifying the terminal hydroxyl group of the PLGA polymer obtained in step 1. with succinic anhydride to obtain the polymer PLGA-COOH whose terminal group is a carboxyl group;
[0037] ③ using the terminal carboxyl group of the PLGA-COOH polymer obtained in ...
Embodiment 2
[0040] This example discloses a targeted drug delivery system modified with 5-boropyridine carboxylic acid and its preparation method. The system of this embodiment includes:
[0041] (a) an aqueous solution of 5-boropyridinecarboxylic acid;
[0042] (b) PLGA-PEI drug-loaded nanoparticles combined with drugs;
[0043] The 5-boropyridinecarboxylic acid has a negative charge, the drug-loaded nanoparticles have a positive charge, and the 5-bororopyridinecarboxylic acid and the drug-loaded nanoparticles are combined through electrostatic interaction.
[0044] The production and application method of this embodiment includes the following steps:
[0045] S1, using two monomers of lactide (LA) and glycolide (GA) for ring-opening copolymerization to obtain a polymer (PLGA), which is then functionalized and reacted with polyethyleneimine (PEI) for amidation reaction, thereby Graft PEI to prepare PLGA-PEI amphiphilic copolymer.
[0046] The specific steps of S1 are:
[0047] ① Usi...
Embodiment 3
[0053] This example discloses a targeted drug delivery system modified with 5-boropyridine carboxylic acid and its preparation method. The system of this embodiment includes:
[0054] (a) an aqueous solution of 5-boropyridinecarboxylic acid;
[0055] (b) PLGA-PEI drug-loaded nanoparticles combined with drugs;
[0056] The 5-boropyridinecarboxylic acid has a negative charge, the drug-loaded nanoparticles have a positive charge, and the 5-bororopyridinecarboxylic acid and the drug-loaded nanoparticles are combined through electrostatic interaction.
[0057] The production and application method of this embodiment includes the following steps:
[0058] S1, using two monomers of lactide (LA) and glycolide (GA) for ring-opening copolymerization to obtain a polymer (PLGA), which is then functionalized and reacted with polyethyleneimine (PEI) for amidation reaction, thereby Graft PEI to prepare PLGA-PEI amphiphilic copolymer.
[0059] The specific steps of S1 are:
[0060] ① Usi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com