Imidazoline derivatives as well as synthesis method and application thereof
A technology of imidazoline derivatives and synthesis methods, applied in chemical instruments and methods, water/sludge/sewage treatment, organic chemistry, etc., can solve the problem of increased concentration of corrosion inhibitors and difficulty in compatibility with various environmental conditions , increase the cost of oilfield sewage treatment and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
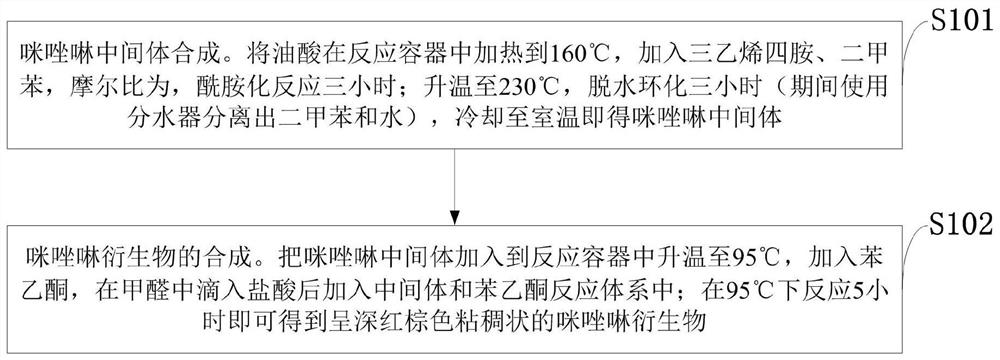
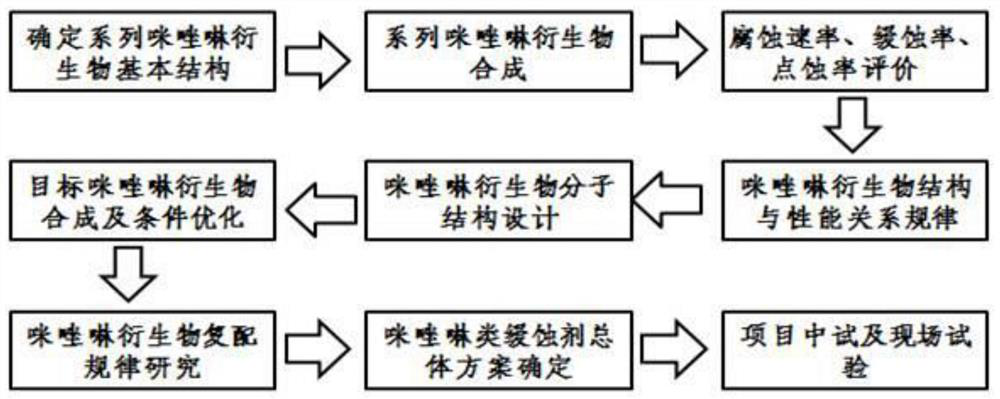
[0040] Such as figure 1 As shown, the synthetic method of the imidazoline derivative provided by the embodiment of the present invention comprises the following steps:
[0041] S101: Synthesis of imidazoline intermediates. Heat oleic acid to 160°C in a reaction vessel, add triethylenetetramine and xylene at a molar ratio of 1:1.2:1.2, and perform amidation reaction for three hours; raise the temperature to 230°C, and perform dehydration and cyclization for three hours (using a few minutes during the period) The water device separates the xylene and water), and cools to room temperature to obtain the imidazoline intermediate.
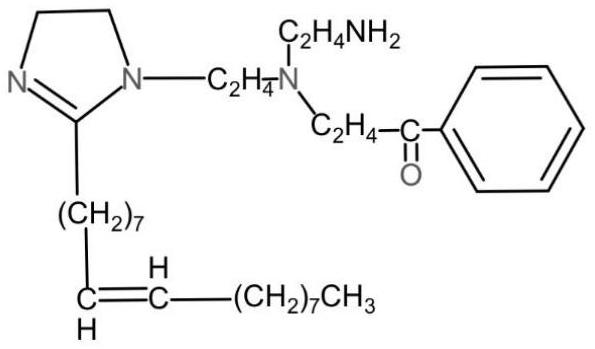
[0042] S102: Synthesis of imidazoline derivatives. Add the imidazoline intermediate to the reaction vessel and raise the temperature to 95°C, add acetophenone, drop hydrochloric acid into the formaldehyde, add the intermediate and acetophenone to the reaction system; react at 95°C for 5 hours to obtain Dark reddish brown viscous imidazoline derivativ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com