Tofacitinib or tofacitinib salt sustained release preparation and preparation method thereof
A sustained-release preparation, tofacitinib technology, applied in medical preparations with non-active ingredients, medical preparations containing active ingredients, pill delivery, etc., can solve incomplete drug release, reduced solubility, and low melting point of sorbitol and other problems to achieve the effect of avoiding the "pan-JAK" inhibition effect and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0083] 1. Prescription
[0084] The compositions of the ingredients in the sustained-release formulations of prescriptions 1-16 are shown in Tables 1-3 below.
[0085] Table 1 Composition of ingredients in the sustained-release preparations of prescriptions 1-6
[0086]
[0087] Table 2 Composition of each ingredient in the sustained-release preparations of prescriptions 7-11
[0088]
[0089] Table 3 Composition of ingredients in the sustained-release preparations of prescriptions 12-16
[0090]
[0091] 2. Preparation method
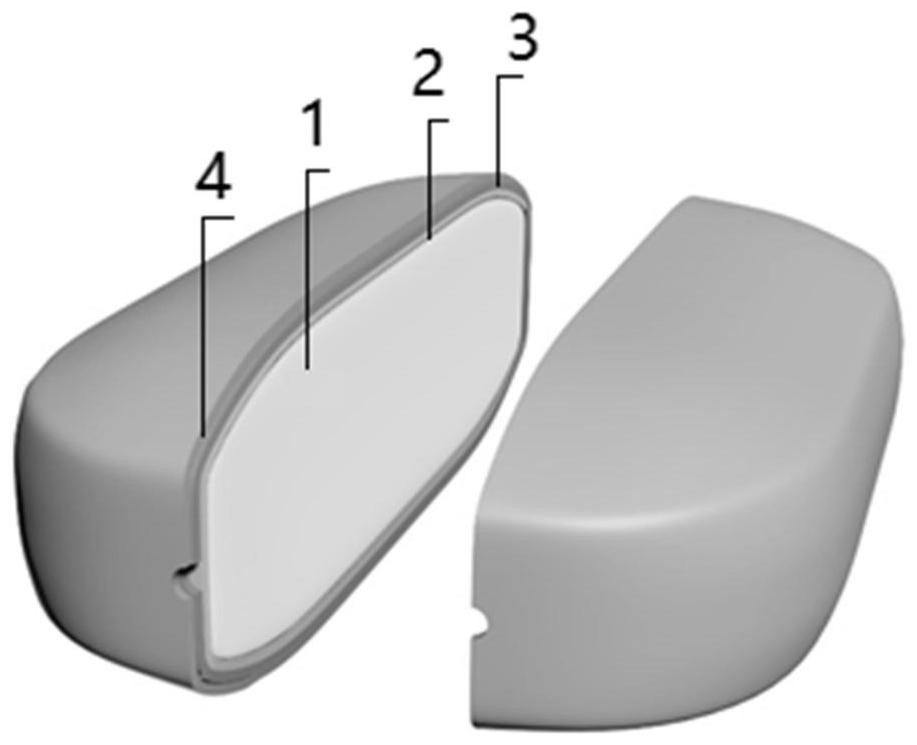
[0092] The sustained-release preparation of tofacitinib or its salt of the present invention comprises the following preparation steps according to the sequence of the preparation process: (1) preparation of the drug-containing composition and the osmotic tablet core; (2) optional sealing isolation coat coating ; (3) release-controlling coating; (4) perforation of the coated tablet; and optionally, (5) aesthetic coating.
[0093] (1) Table...
experiment Embodiment 1
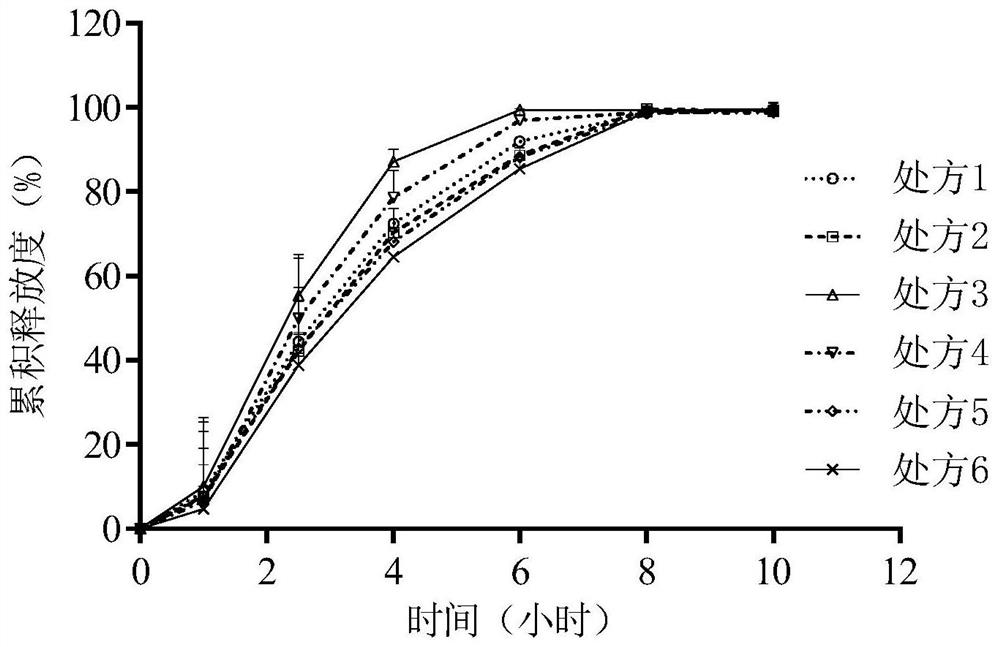
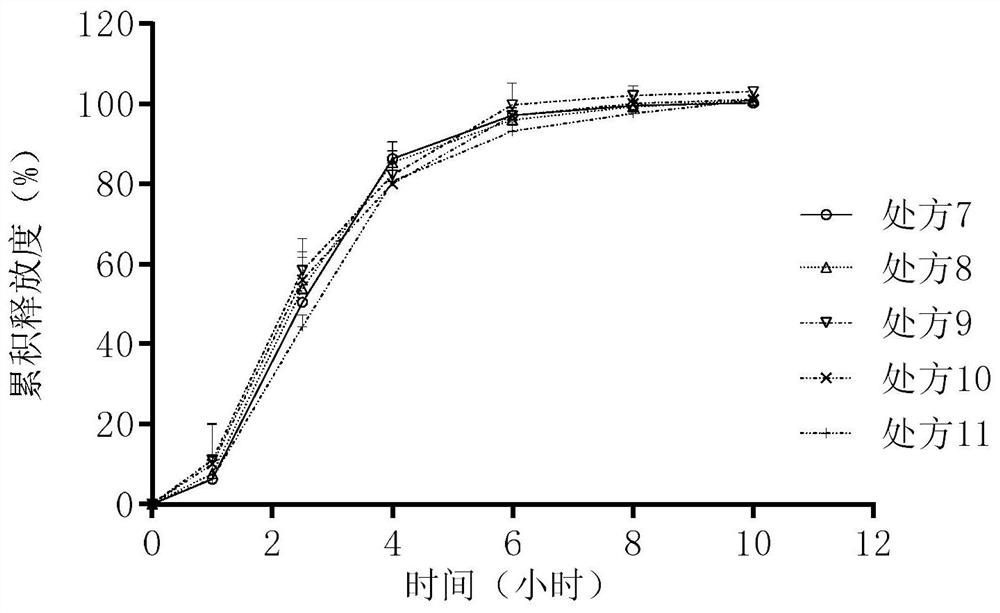
[0113] Experimental Example 1: Determination of the release rate of prescriptions 1 to 16
[0114] Using the second method device of the dissolution and release test method (general rule 0931), put the osmotic pump tablet in the sedimentation basket, use 900mL of pH6.8 phosphate buffer as the release medium, and operate according to the law at a speed of 50rpm. After 1, 2.5 , 4, 6, 8, and 10 hours each take 5 mL of the solution, and replenish the release medium at the same temperature and volume in time. Get sample solution, centrifuge (8000rpm, 10min), get supernatant as need testing solution. Another appropriate amount of tofacitinib citrate reference substance was taken, accurately weighed, dissolved in a release medium and quantitatively diluted to make about 12 μg of tofacitinib citrate per 1 ml, as a reference solution. According to the high-performance liquid chromatography (general rule 0512), each 10 μ l of the above-mentioned reference substance solution and need te...
experiment Embodiment 2
[0123] Experimental Example 2: Comparison of Release Behavior of Prescription 11 and Commercially Available Sustained-release Preparations in Different pH Media
[0124] Due to the solubility of drugs under different pH conditions, the hydration, swelling, and erosion rates of key excipients that control drug release behavior may be different. Therefore, the drug release behavior of formulation 11 in Preparation Example 1 and the commercially available sustained-release preparation in release media with different pH values was investigated.
[0125] Using the second method device of the dissolution and release assay method (general rule 0931), put the osmotic pump tablet in the sedimentation basket, and use 900mL of release media with different pH values (including ①pH1.2 hydrochloric acid solution; ②pH4.5 phosphate buffer; ③pH6.8 phosphate buffer solution; ④pH7.4 phosphate buffer solution), the rotating speed is 50rpm, operate according to the law, after 1, 2.5, 4, 6, 8, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com