A deoxyglucose-modified folic acid derivative and its synthesis and application
A technology of deoxyglucose and folic acid derivatives, applied in the field of biomedicine, can solve the problems of poor curative effect, easy to cause immune reaction, uneven particle size of liposomes, etc., and achieve the effect of correct structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
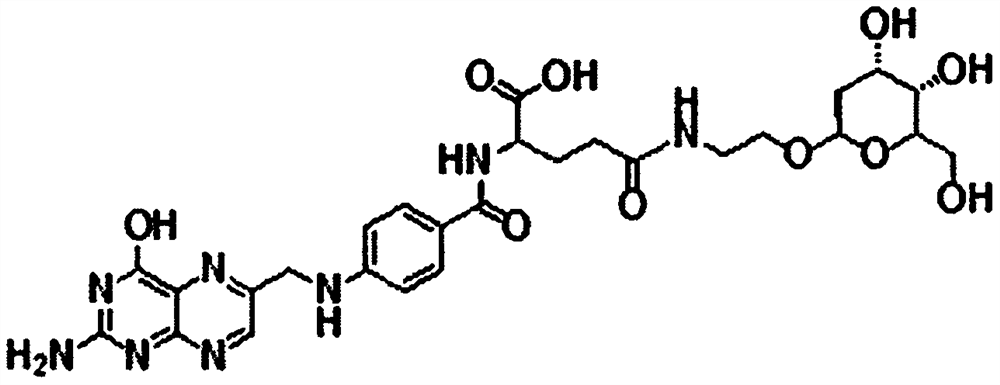
[0037]This example provides a deoxyglucose-modified folic acid derivative. The folic acid derivative (FA-2-DG) is formed by linking deoxyglucose and folic acid via aminoethanol, wherein the carboxyl group of folic acid is connected with the amino group of aminoethanol to form The amide bond, the hydroxyl group of aminoethanol and the hydroxyl group of deoxyglucose are connected to form a glycosidic bond. The chemical structural formula of the FA-2-DG is as figure 1 shown.
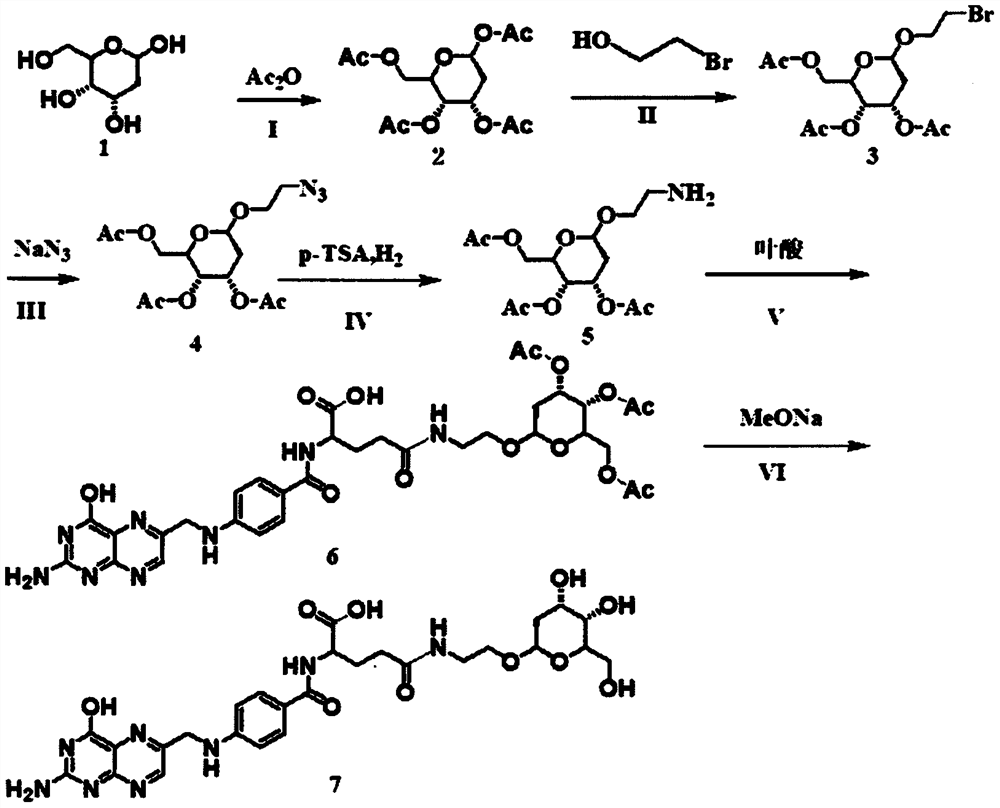
[0038] Deoxyglucose has multiple hydroxyl groups. During the reaction, the hydroxyl groups have high reactivity, so they need to be protected with acetyl groups before the reaction. The acetyl-protected reaction proceeds from deoxyglucose and acetic anhydride in anhydrous pyridine. The amino group of aminoethanol will also have relatively high reactivity during the reaction process, so aminoethanol is not used directly in the reaction process, but 2-bromoethanol is used to react with acetyl deoxyglucose f...
Embodiment 2
[0067] This embodiment provides a targeted anti-tumor nanoparticle, the nanoparticle is composed of the above-mentioned folic acid derivative and the anti-tumor drug cisplatin linked by coordination bonds. To prepare the complex of FA-2-DG and cisplatin, mix the aqueous solution of cisplatin and FA-2-DG of the same concentration and stir at room temperature. After two hours, cisplatin and FA-2-DG are formed as Figure 5 The coordination compound (FA-2-DG-Pt) shown. The mixed solution was injected into HPLC-MS analysis, and the formation of the complex was confirmed. For mass spectrometry results, see Figure 6 .
[0068] The morphological characterization techniques of atomic force microscopy, transmission electron microscopy and scanning electron microscopy all confirmed that FA-2-DG itself can self-assemble to form nanoparticles, and form a coordination bond with cisplatin, and obtain FA-2-DG-Pt after drug loading. It can also self-assemble into nanostructures.
[0069] ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com