Immobilization method of beta-galactosidase and application of beta-galactosidase in preparation of low-lactose milk
A technology of galactosidase and immobilized enzyme, applied in the field of immobilized enzyme biocatalysis, can solve the problems of unsuitable food industry, toxicity and the like, and achieve the effects of good industrial application value, high hydrolysis rate and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Example 1: Optimization of the immobilization conditions of β-galactosidase
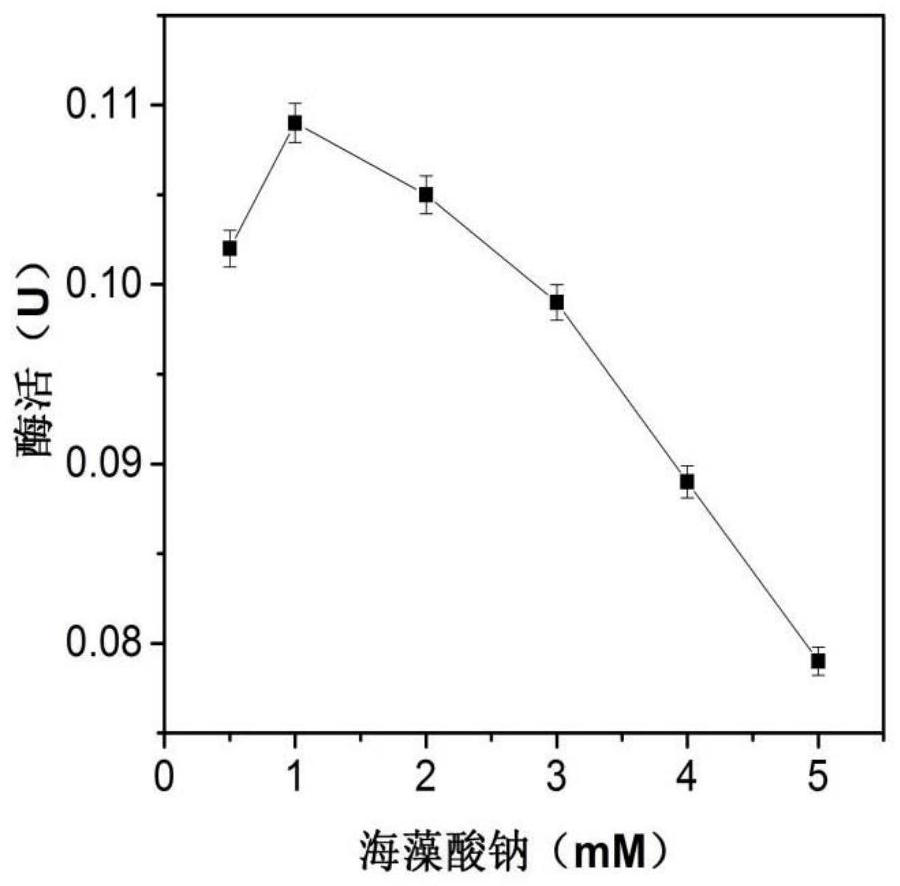
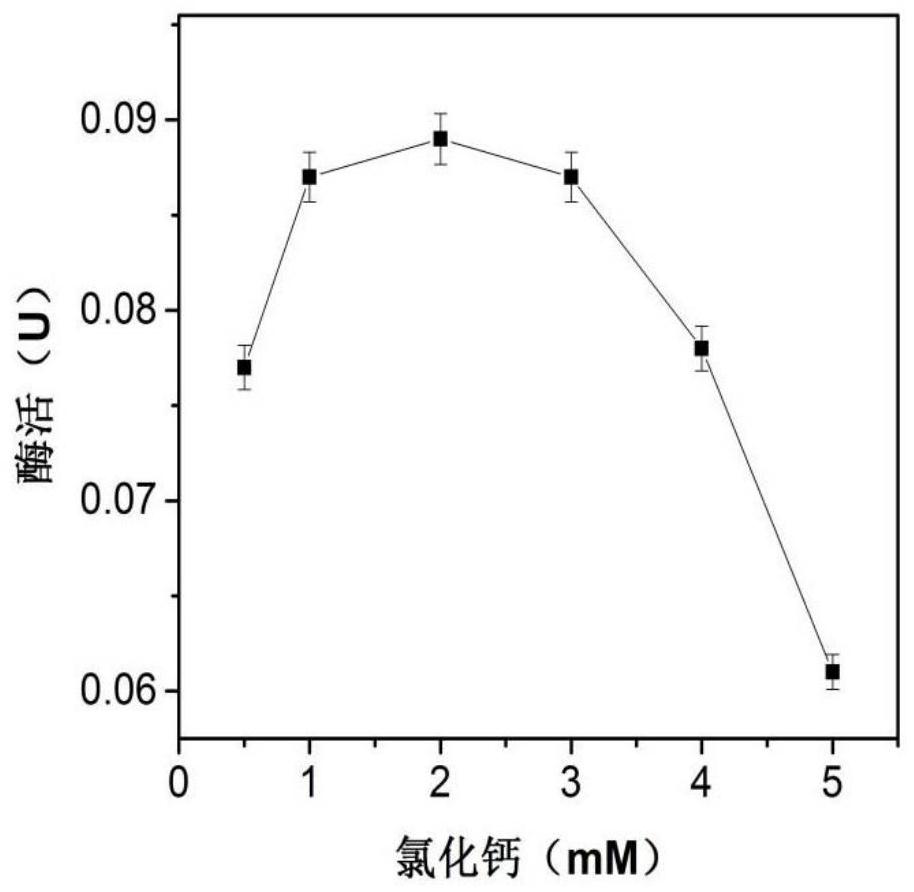
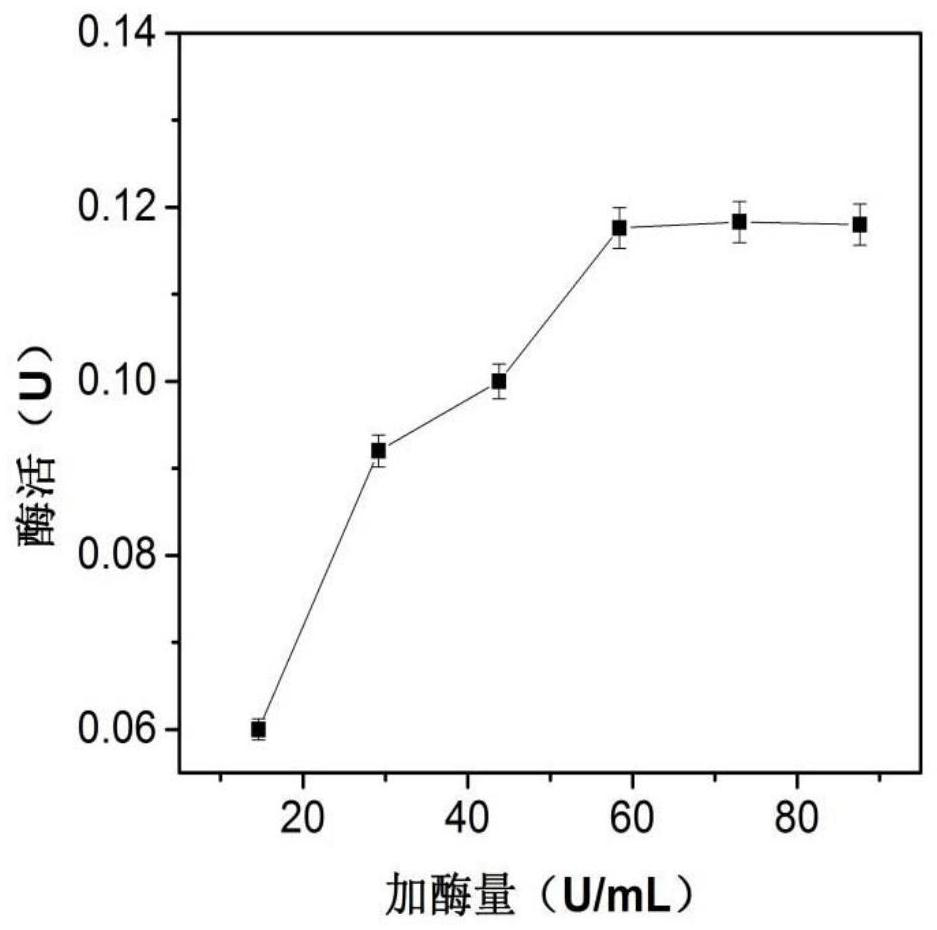
[0027] Now use phosphate buffer to prepare 23.2mM sodium alginate solution, add 1mL enzyme solution (enzyme concentration 0.04mg / mL) to 1mL sodium alginate solution, put it in an ice bath (0°C), and run at 50-60rpm stirring speed. Use a syringe to draw the mixed solution and add it dropwise to 20mL 90.1mM CaCl at a rate of 2d / s 2 In solution, calcium alginate beads were formed. After obtaining the immobilized enzyme particles, continue to stand at room temperature for 1-2 hours to make them harden, and finally wash the particles with 50mM pH 6.5 phosphate buffer to remove unbound enzyme molecules and obtain the immobilized enzyme of β-galactosidase , stored in the refrigerator at 4°C. Among them, the concentration of sodium alginate, CaCl 2 Concentration and initial enzyme amount need to be optimized, such as figure 1 , figure 2 , image 3 As shown, the results show that when the conce...
Embodiment 2
[0028] Example 2: Determination of immobilized β-galactosidase activity
[0029] β-galactosidase can specifically hydrolyze the β-1,4-glycosidic bond in o-nitrophenyl-β-D glucopyranoside (oNPG), releasing o-nitrophenyl (oNP) and glucose, oNP It is yellow under alkaline conditions. Under a certain range of conditions, the concentration of oNP and its OD 420 There is a certain linear relationship between the absorbance value. With oNPG as the substrate, the enzyme activity unit (U) definition: under the optimal action conditions, the amount of enzyme required to catalyze the hydrolysis of oNPG to generate 1umol oNP per minute is 1 U. The measured oNP curve of o-nitrobenzene is: Y=2.1286X-0.0023, R 2 = 0.9999. Among them, Y is OD 420 The absorbance value of X is the concentration of oNP in the system (mM).
[0030] With 50mM, pH 6.5 citrate-Na 2 HPO 4 Prepare 50mM oNPG solution in the buffer as the substrate, mix 1mL of the substrate solution with 0.12U / mL immobilized enzy...
Embodiment 3
[0031] Embodiment 3: Optimum temperature, pH determination of immobilized enzyme
[0032]Set the temperature gradient (50°C, 55°C, 60°C, 65°C, 70°C, 75°C), set three parallels for each treatment, measure the enzyme activity at each temperature under the condition of pH 6.5, and draw the optimum temperature curve. Such as Figure 4 As shown, the optimum reaction temperature of the immobilized enzyme was found to be 65°C, which was the same as that of the free enzyme.
[0033] Prepare 50mM pH5-7.5 pH series buffers, dissolve the substrate oNPG in the pH series buffers, set up three parallels for each, measure enzyme activities at different pHs at 65°C, and draw the optimum pH curve. Such as Figure 5 As shown, the optimum pH of the immobilized enzyme was measured to be 6.5, which was the same as that of the free enzyme.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com