Thermal activation delayed fluorescence material capable of being processed by solution, preparation method and application thereof
A technology of thermally activated delayed and fluorescent materials, applied in the fields of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc. Properties, preparation and purification methods are simple, and the effect of improving properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
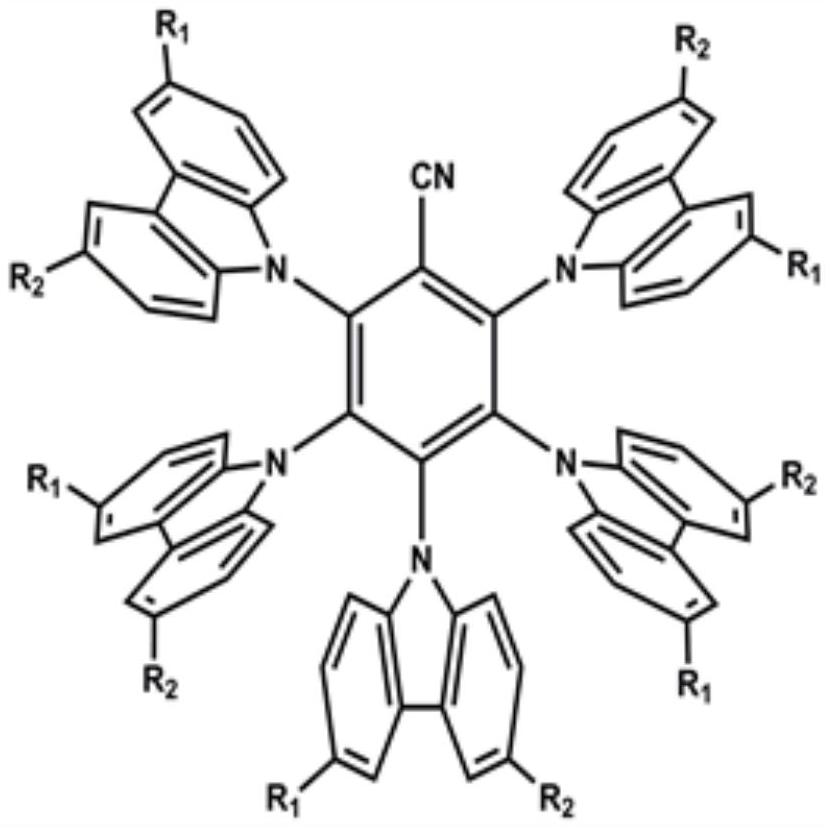
[0032] Embodiment 1: the synthesis of compound C1
[0033] Step 1, Synthesis of 3,6-bis(4-(9H-carbazol-9-yl)phenoxy)-9H-carbazole in peripheral dendrons
[0034] Add 3,6-dihydroxy-9-hydrogen-carbazole (10g, 25.12mmol), cesium carbonate (4.5g, 13.84mmol), 9-(4-iodophenyl)-9H-carbazole into a 500mL reaction flask (15g, 54.94mmol), N,N-dimethylformamide (200mL), under the protection of nitrogen, react at 100°C for 4h. After the reaction was finished, cool to room temperature, add a large amount of water to stir, and filter with suction to obtain the crude product. Then, it was purified by column chromatography to obtain 3,6-bis(4-(9H-carbazol-9-yl)phenoxy)-9H-carbazole as a white solid with a yield of 70%.
[0035] Step 2, Synthesis of C1
[0036] In dry tetrahydrofuran (THF, 20 mL) was added 3,6-bis(4-(9H-carbazol-9-yl)phenoxy)-9H-carbazole (3.26 g, 3.15 mmol), sodium hydride (0.5 g, 20.3mmol), stirred at room temperature for 0.5h, then added 2,3,4,5,6-pentafluorobenzonitril...
Embodiment 2
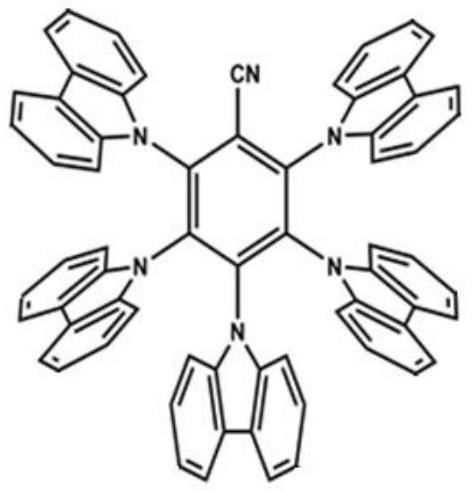
[0041] Embodiment 2: Synthesis of C2
[0042] The 3,6-dihydroxy-9-hydrogen-carbazole that reacts with 9-(4-iodophenyl)-9H-carbazole in Example 1 is replaced with 3-hydroxyl carbazole, and through the same process as in Example 1 Synthetic method, the product C2 was obtained. Yield 70%.
[0043] Mass Spectrum: 2214.76.
[0044] Elemental analysis results: C: 85.11, H: 4.32, N: 6.95, O: 3.61.
Embodiment 3
[0045] Embodiment 3: Synthesis of C3
[0046]In the above example 1, the 9-(4-iodophenyl)-9H-carbazole reacted with 3,6-dihydroxy-9-hydrogen-carbazole was replaced by 4-iodo-N, N-diphenylaniline , through the same synthesis method as in Example 1, the product C3 was obtained. Yield 71%.
[0047] Mass spectrum: 3520.33.
[0048] Elemental analysis results: C: 84.23, H: 4.87, N: 6.36, O: 4.56.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com