Method for modifying carbonyl reductase stereoselectivity, carbonyl reductase mutant and application
A stereoselective and reductase technology, applied in the direction of microbial-based methods, oxidoreductases, applications, etc., can solve the problems of carbonyl reductase structural differences, low protein structure similarity, and weak reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
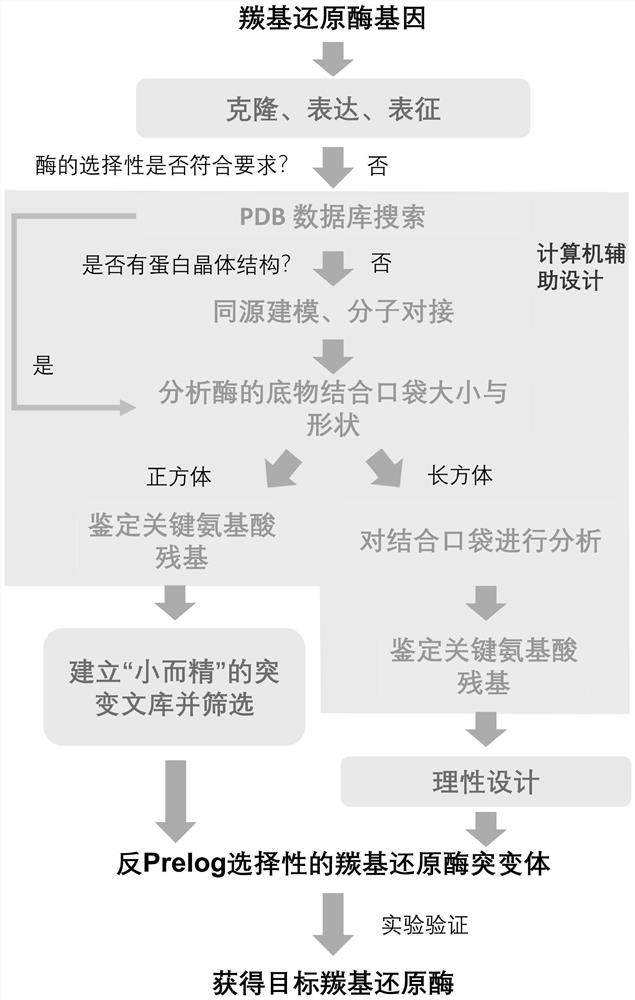
[0040] Example 1: Establishing a method for predicting stereoselectivity of carbonyl reductase
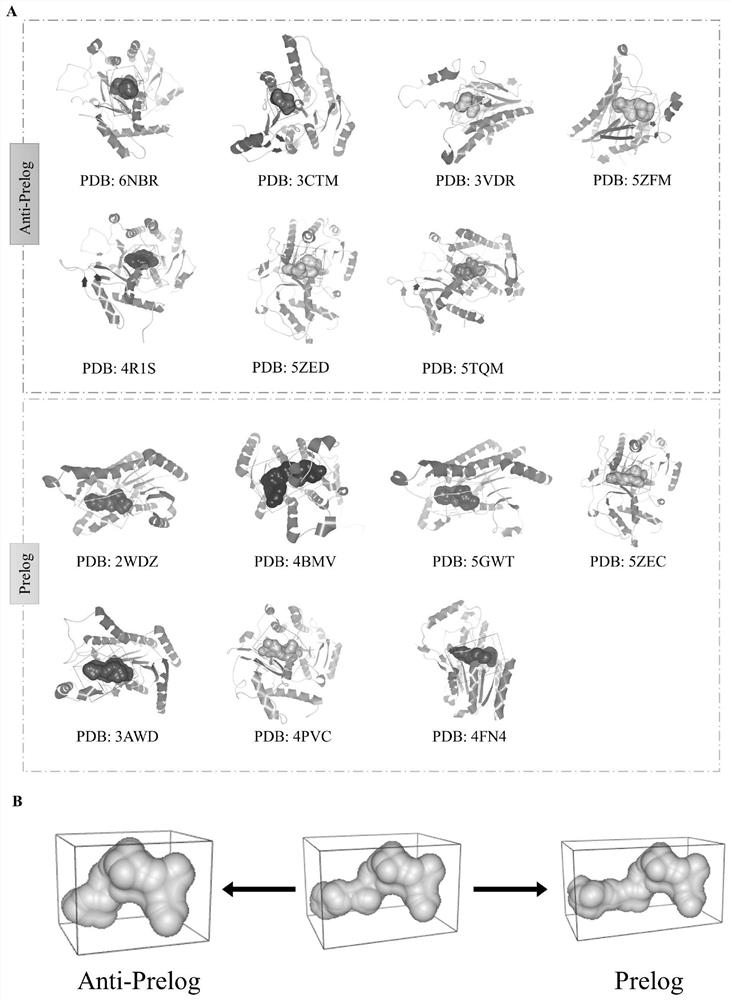
[0041] refer to figure 1 , search the crystal structure of carbonyl reductase in the PDB database, import its three-dimensional model into CaverAnalyst 2.0, select the pocket analysis of the software, and set the size of the probe to The size and shape of the substrate binding pocket of the enzyme were analyzed, and the results were as follows: figure 2 Shown in (A). The outer shape of the substrate-binding pockets of the reported enzymes following the anti-Prelog rule (PDB: 3CTM, 5ZFM, 4R1S, 5ZED, 5TQW, 3VDR, and 6NBR) is like a cube (six equilateral square regular entities), while following Prelog-ruled enzymes are more cuboid-like (PDB: 2WDZ, 4BMV, 5GWT, 3AWD, 4PVC, 4FN4 and 5ZEC). The sequences of the 7 anti-Prelog enzymes were compared with the sequences of the 7 Prelog enzymes, and the results are shown in Table 1. After comparison, it is concluded that anti-Prelog enzy...
Embodiment 2
[0044] Example 2: Computer Prediction of Stereoselectivity of Eleven Unexplored Carbonyl Reductases
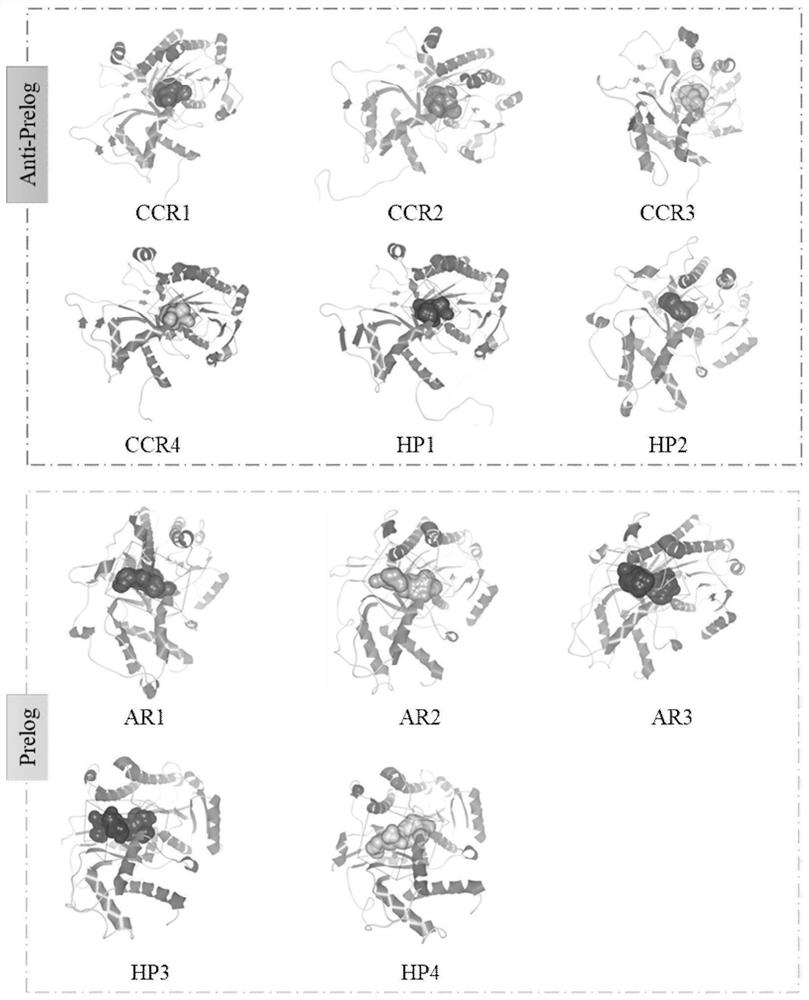
[0045] According to the rules summarized in Example 1, undeveloped short-chain dehydrogenases with a sequence similarity of 20-40% to KmCR and an amino acid sequence length of 320-350 were searched in the NCBI database. Homology modeling was carried out on them respectively, and the size, shape and amino acid residues at key sites of the enzyme substrate binding pocket were observed. The result is as Figure 10 As shown, the shape of the binding pocket of CCR1, CCR2, CCR3, CCR4, HP1, HP2 is similar to a cube, while the shape of the binding pocket of AR1, HP3, AR2, AR3 and HP4 is similar to a cuboid. By comparing the amino acids at the key sites (A1, A2, A3, B1, B2), the results are shown in Table 2, 6 sequences satisfy the law of inverse Prelog, and 5 sequences satisfy the law of Prelog.
[0046] Table 2 Predicted stereoselectivity of an unexplored carbonyl reductase towards...
Embodiment 3
[0049] Example 3: Cloning of wild-type carbonyl reductase KmCR gene and construction of its recombinant expression vector
[0050] (1) Target gene cloning
[0051] Using Kluyveromyces marxianus (K.marxianus) ZJB14056 (Depository Number: ATCC36534) genomic DNA as a template, through the upstream primer: 5'-TAAGAAGGAGATATA CCATGG ATGACATTTACAGTGGTGACAG-3', downstream primer 5'-GTGGTGGTGGTGGTG CTCGAG TTACCCACGGTACGCGCCCA-3' was amplified by PCR to obtain the amplified product. Take 5 μL of PCR amplification products for agarose gel electrophoresis test. See the test results Figure 4 Swimming lanes 1, 2, and 3 have clear electrophoresis bands with a size of about 1000bp, which is consistent with the theoretical value (1038bp). PCR system (total volume 100 μL): 50 μL 2×PhantaMax buffer, 40 μL double distilled water, 2 μL dNTP Mix2, 2 μL dNTP mixture (10 mM each), 2 μL each of upstream and downstream primers, 2 μL genomic DNA, Phanta Max Super-Fidelity DNA polymerase 2 μL. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com