Respiratory tract virus nucleic acid sampling test kit and application thereof
A virus nucleic acid and respiratory technology, applied in the field of biomedicine, can solve the hidden dangers of epidemic prevention and control, insufficient sample collection and other problems, and achieve the effect of reducing the discomfort of sampling, convenient sampling, and improving the detection rate of nucleic acids.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
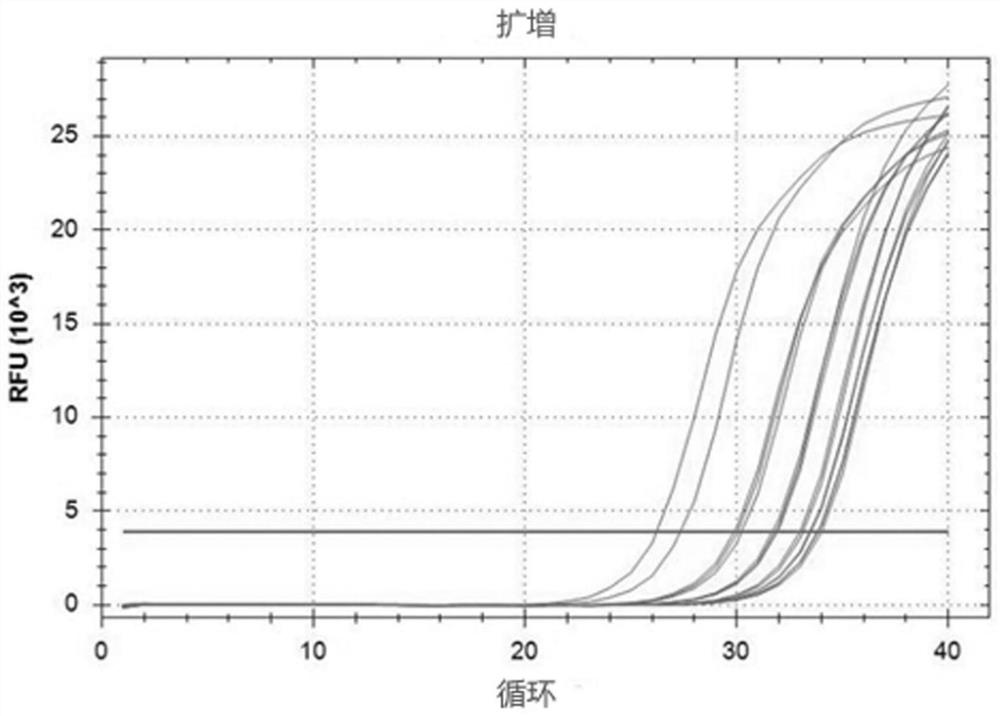
[0028] This embodiment provides a sampling kit for respiratory virus nucleic acid detection, including nasal spray and sample preservation solution 1, the nasal spray comprises: 0.9% sodium chloride, 0.5% sodium pyruvate according to the composition of weight percentage , 0.01% pyruvate, 0.1% Tween 80, 0.01% glyceryl monostearate, and the balance is sterile water; the sample preservation solution-comprises according to weight percentage: 0.12% glucose, 0.02 % calcium chloride, 0.05% potassium chloride, 0.01% disodium hydrogen phosphate, 0.015% isoleucine, 0.01% leucine, 0.015% lysine hydrochloride, the balance is sterile physiological brine.
Embodiment 2
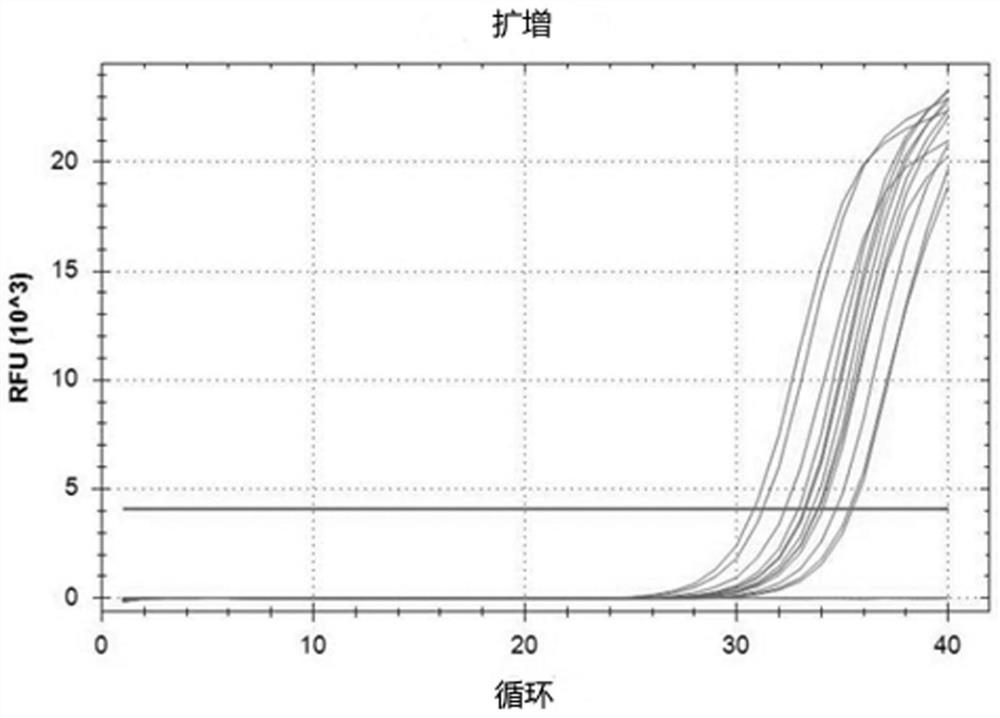
[0030] This embodiment provides a sampling kit for respiratory virus nucleic acid detection, including nasal spray and sample preservation solution 1, the nasal spray comprises: 3% sodium chloride, 0.1% sodium pyruvate according to the composition of weight percentage , 0.1% pyruvate, 0.01% Tween 80, 0.1% glyceryl monostearate, and the balance is sterile water; the sample preservation solution-comprises according to weight percentage: 0.08% glucose, 0.03 % calcium chloride, 0.03% potassium chloride, 0.015% disodium hydrogen phosphate, 0.01% isoleucine, 0.015% leucine, 0.01% lysine hydrochloride, the balance is sterile physiological brine.
Embodiment 3
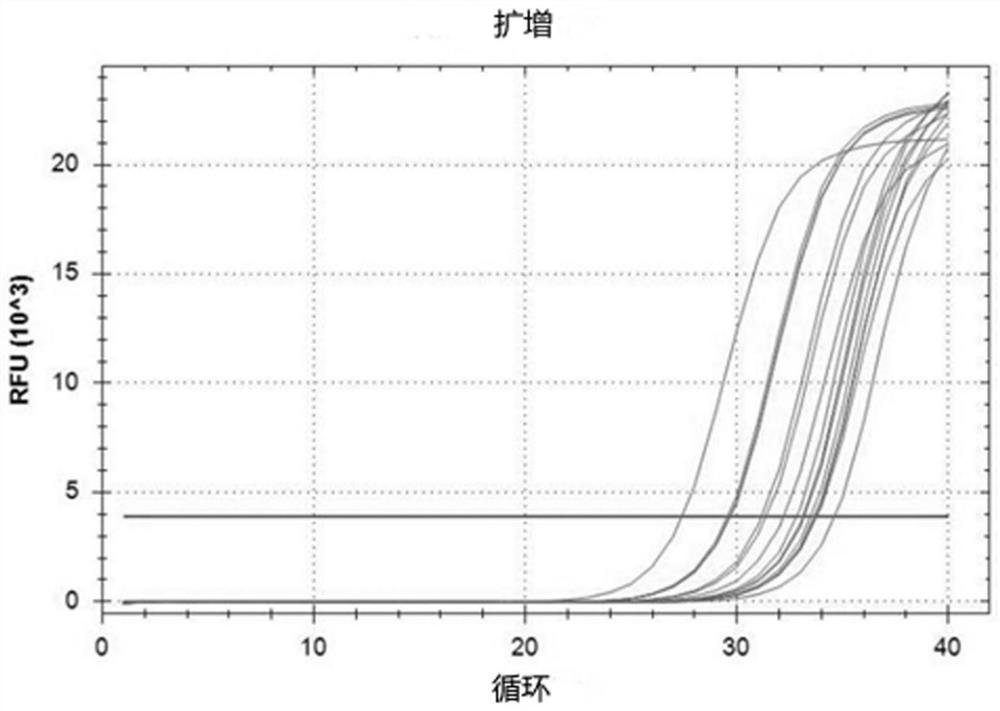
[0032] This embodiment provides a sampling kit for respiratory virus nucleic acid detection, including nasal spray and sample preservation solution 1, the nasal spray comprises: 1.2% sodium chloride, 0.3% sodium pyruvate according to the composition of weight percentage , 0.05% pyruvate, 0.05% Tween 80, 0.05% glyceryl monostearate, and the balance is sterile water; the sample preservation solution-comprises according to weight percentage: 0.10% glucose, 0.03 % calcium chloride, 0.04% potassium chloride, 0.012% disodium hydrogen phosphate, 0.013% isoleucine, 0.012% leucine, 0.012% lysine hydrochloride, the balance is sterile physiological brine.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com