Fluorine-containing heat transfer fluid, and preparation method and application thereof
A technology of heat transfer fluid and fluorine-containing alkoxy group, which is applied in the fields of ether preparation, ester reaction preparation of ether, lighting and heating equipment, etc., which can solve the long residence time, difficult synthesis, and inability to meet the operation requirements of devices loaded with heat transfer fluid And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
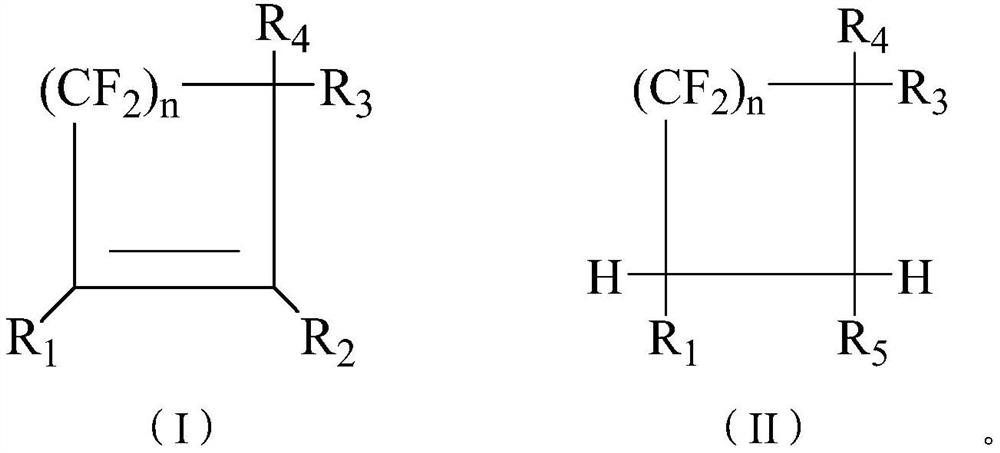
[0080] The present application also provides a method for preparing a fluorine-containing heat transfer fluid, which comprises: combining any one or several structural compounds in formula (I) and any one or several structural compounds in formula (II) in the form of any substance The proportion of the amount is mixed to obtain the fluorine-containing heat transfer fluid;
[0081] The present application also provides a method for preparing a fluorine-containing heat transfer fluid, which includes: obtaining the chlorine-containing formula (I) by using a first nucleophilic substitution reaction of a halogenated cyclic olefin with an alcohol to obtain the fluorine-containing heat transfer fluid.
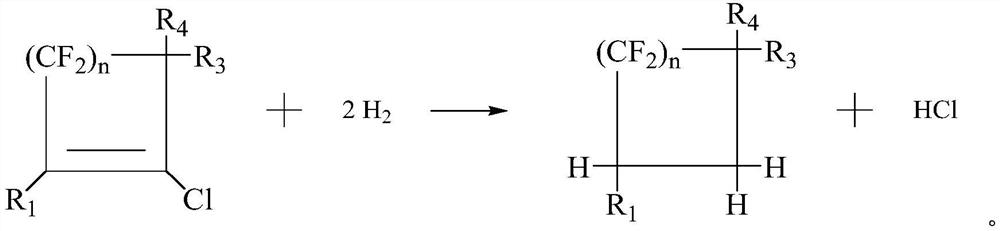
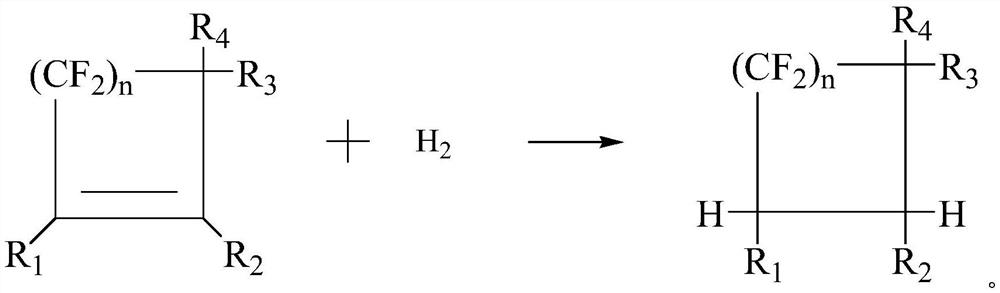
[0082] In a specific embodiment, the fluorine-chlorine exchange reaction of the chlorine-containing formula (I) with an alkali metal fluoride yields the fluorine-containing heat transfer fluid.
[0083] In a specific embodiment, the second nucleophilic substitution reaction of the flu...
Embodiment 1
[0103] Preparation of 1-(2,2,3,3-tetrafluoropropoxy)-2-chloro-3,3,4,4,5,5-hexafluorocyclopentene
[0104] method one:
[0105]
[0106] Under normal pressure and stirring conditions, in a 500 ml glass flask equipped with a condenser tube and a bubbler (to make the reaction at normal pressure), add 2,2,3,3-tetrafluoropropanol, potassium hydroxide and 1- Chlorohptafluorocyclopentene (0.4mol), its molar ratio m(2,2,3,3-tetrafluoropropanol):m(potassium hydroxide):m(1-chloroheptafluorocyclopentene)=2 : 1: 1, the reaction temperature is 20°C, and the reaction time is 0.5 hours. After the reaction, potassium fluoride and potassium hydroxide solids were removed by filtration, and then the liquid organic phase was distilled to obtain 1-(2,2,3,3-tetrafluoropropoxy)-2-chloro 3,3, 4,4,5,5-hexafluorocyclopentene (boiling point is 111-113°C / 760mmHg), analyzed by gas chromatography, the following results are obtained: the yield is 90.4%, the purity is 98.6%, and GC-Ms analysis conform ...
Embodiment 2
[0111] Preparation of 1,3-bis(2,2,3,3-tetrafluoropropoxy)-2-chloro-3,4,4,5,5-pentafluorocyclopentene
[0112] method one:
[0113]
[0114] Under normal pressure and stirring conditions, in a 500 ml glass flask equipped with a condenser tube and a bubbler (to make the reaction at normal pressure), add 2,2,3,3-tetrafluoropropanol, potassium hydroxide and 1- Chlorohptafluorocyclopentene (0.4mol), its molar ratio m(2,2,3,3-tetrafluoropropanol):m(potassium hydroxide):m(1-chloroheptafluorocyclopentene)=5 :2:1, reaction temperature 20°C, reaction time 1 hour. After the reaction, potassium fluoride and potassium hydroxide were removed by filtration, and then the liquid organic phase was distilled to obtain 1,3-bis(2,2,3,3-tetrafluoropropoxy)-2-chloro 3 , 4,4,5,5-pentafluorocyclopentene (boiling point is 196-198 ° C / 760mmHg), using gas chromatography analysis, the following results are obtained: the yield is 85.1%, the purity is 97.2%, and GC-Ms The analysis matches the specifie...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com