Pyrazine compound and preparation method thereof
A compound, pyrazine technology
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] The synthesis of embodiment 1 compound OLB-1
[0061]
[0062] Step (1): Compound 1-0 (15.0g, 110.3mmol) was dissolved in glacial acetic acid (150ml), hydrogen peroxide (30%, 12.5ml, 110.2mmol) was added dropwise at 70°C, and the reaction was continued overnight. After the reaction, cool, dilute with aqueous sodium hydroxide solution (50%), extract with dichloromethane, dry over anhydrous sodium sulfate, filter, and concentrate to obtain the crude compound directly dissolved in acetic anhydride (30ml), react at 107°C for 3 hours, and the reaction ends Afterwards, cooling, concentrating, pouring into ice water to dilute, adjusting the pH to greater than 10 with sodium hydroxide solution, stirring overnight, extracting with dichloromethane, drying over anhydrous sodium sulfate, filtering, concentrating, and silica gel column chromatography to obtain the product 1-1 (6.8 g, 41%). 1 H NMR (400MHz, DMSO-d 6 )δ5.39(s,1H), 3.81(s,2H), 2.42(s,3H), 2.42(s,3H), 2.41(s,3H). ...
Embodiment 2
[0069] The synthesis of embodiment 2 compound OLB-2
[0070]
[0071] Step (1): Compound 1-0 (20g, 147mmol), N-bromosuccinimide (26.7g, 150mmol) and benzoyl peroxide (50mg, 0.2mmol) were dissolved in carbon tetrachloride ( 70ml), under the irradiation of an incandescent lamp, reflux reaction for 10 hours, after the reaction, filter and concentrate to obtain the crude product 1-2, which is directly put into the next reaction. 1 H NMR (400MHz, CDCl 3 )δ4.54(s,2H), 2.57(s,1H), 2.50(s,1H), 2.49(s,1H).
[0072]
[0073] Step (2): Compound 1-2 (17.5g, 82mmol), potassium phthalimide (21.0g, 110mmol) and sodium iodide (0.5g, 3.3mmol) were dissolved in N,N-dimethyl methyl formamide (100ml), stirred at 95°C for 2 hours. After the reaction, filter, pour the filtrate into ice water to obtain a white precipitate, filter with suction, and recrystallize the filter cake with ethanol to obtain product 1-3 (18.7g, 81%). 1 H NMR (400MHz, DMSO-d 6 )δ8.01–7.79 (m, 4H), 4.90 (s, 2H), 2.5...
Embodiment 3
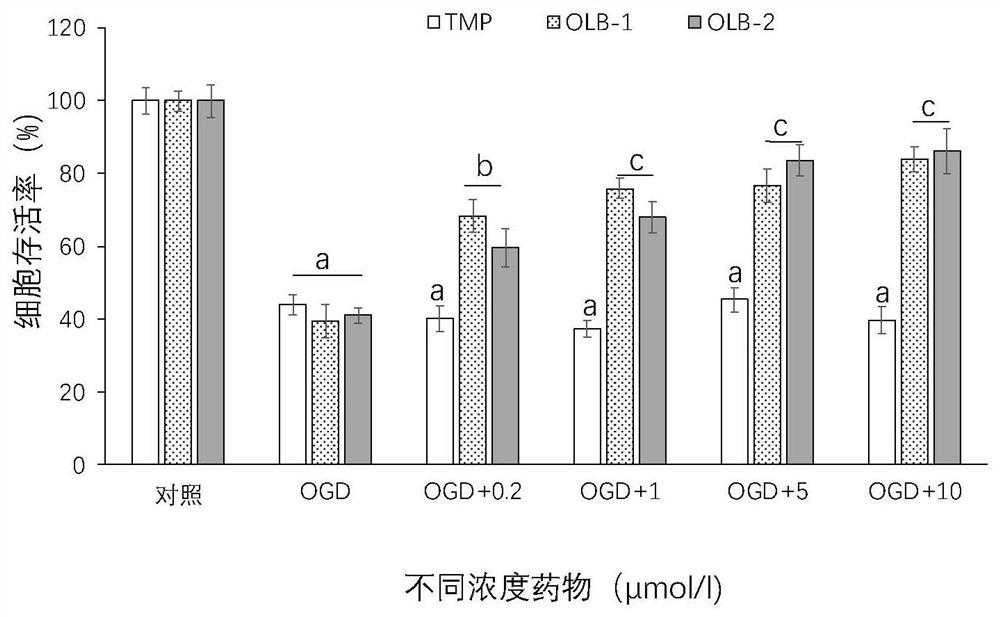
[0081] Example 3: OLB-1 and OLB-2 significantly reduce SH-SY5Y cell death caused by OGD
[0082] MTT method was used to detect and evaluate the neuroprotective effect of TMP (tetramethylmethylpyrazine) and its derivatives. Cultivate cells, collect logarithmic phase cells, adjust the concentration of cell suspension, add drugs and incubate with OGD for 4 hours, add MTT-containing culture medium, incubate for 4 hours, carefully suck out the culture medium in the wells, and add 150 μl dimethyl sulfoxide to each well (DMSO), shake at a low speed on a shaker for 10 minutes to fully dissolve the crystals, measure the absorbance of each well at the OD (absorbance) value of 490nm in the enzyme-linked immunosorbent assay instrument (set zero adjustment wells at the same time (medium, MTT, dimethylformazol) base sulfoxide), control wells (cells, drug dissolution medium at the same concentration, culture medium, MTT, dimethyl sulfoxide)). Data are expressed as mean ± SEM; n = 8 for each ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com