Benzo-pyronine near-infrared fluorescent dye as well as preparation method and application thereof
A benzene ring and alkyl technology, applied in the field of near-infrared fluorescent dyes based on benzopyronine and its preparation, can solve the problems of small Stokes shift, limited application, detection error, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064]
[0065] Compound II-1 was prepared from m-N,N-diethyl-aminophenol according to the literature (Imaging Science and Photochemistry, 2015, 33(2), 154–160), with a total yield of 40%.
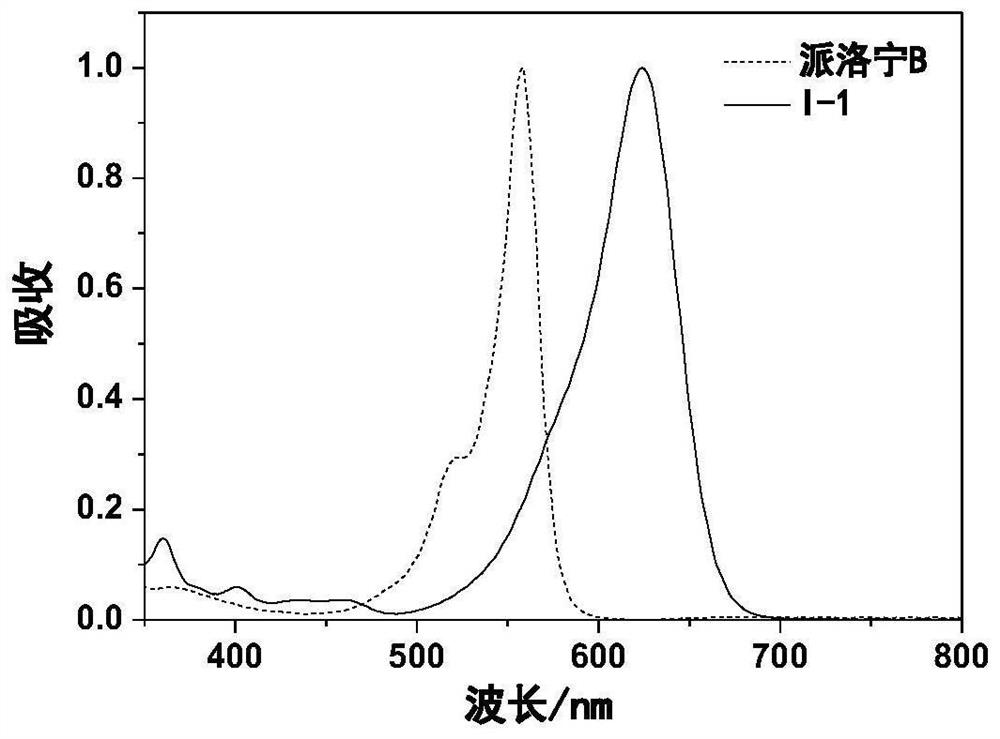
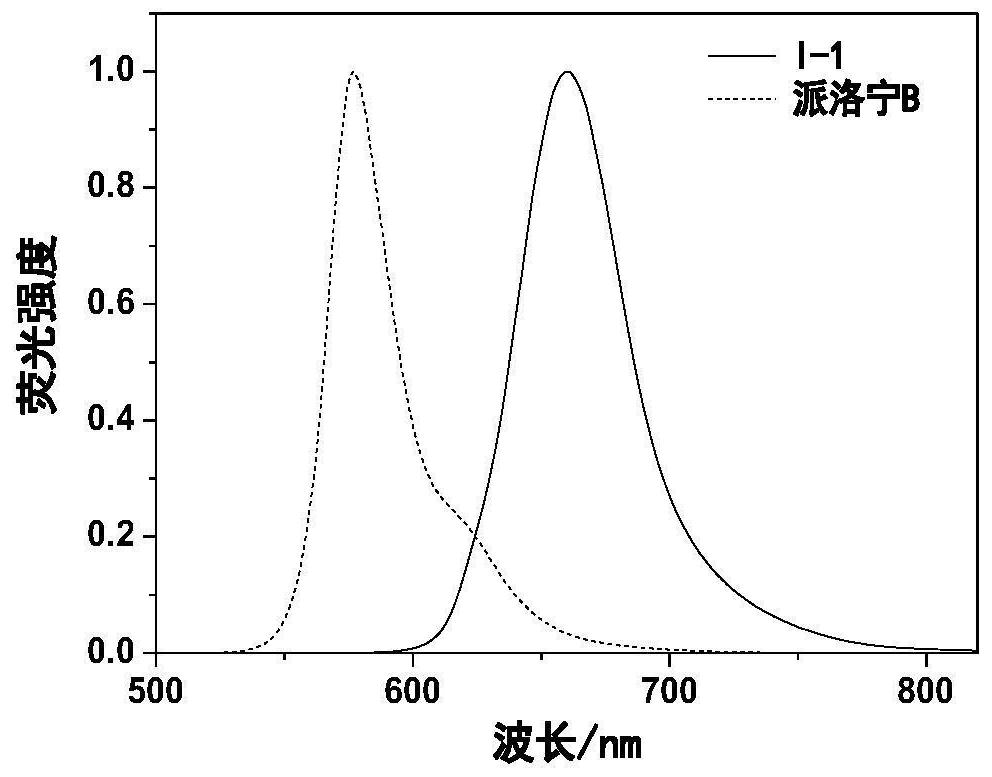
[0066] Dissolve 0.26g (0.001mol) of compound II-1 and 0.27g (0.002mol) of compound III-1 in 5 ml of acetic acid, add 0.1 ml of perchloric acid (mass percentage concentration is 70%), and heat to 150°C, react for 2h. After cooling, add 1 milliliter of perchloric acid (mass percentage concentration is 70%), then slowly add distilled water dropwise, precipitate solid, filter, vacuum dry, column chromatography purification and separation to obtain 0.13g compound I-1, yield 29.3% . ESI MS: m / z, 346.2 (M+H). lambda ab. max / nm=624nm,λ em max / nm=688nm,Ф f = 0.66. Compound I-1 in dichloromethane, compared with Pyronin B, has a red-shift of 68nm in absorption, a red-shift of 92nm in fluorescence, a 1-fold increase in Stokes shift, and a 1.5-fold increase in fluorescence quantum yield. ...
Embodiment 2
[0068]
[0069] Dissolve 0.26g (0.001mol) of compound II-1 and 0.16g (0.001mol) of compound III-2 in 10ml of ethanol, add 2ml of concentrated sulfuric acid, heat to 80°C, and react for 3h. After cooling, 0.5 ml of perchloric acid (mass percent concentration: 70%) was added, and distilled water was slowly added dropwise to precipitate a solid, which was filtered, vacuum-dried, and purified by column chromatography to obtain 0.16 g of compound I-2 with a yield of 34.0%. ESI MS: m / z, 371.2. lambda ab. max / nm=625nm,λ em max / nm=686nm,Ф f = 0.65.
Embodiment 3
[0071]
[0072] Dissolve 0.23g (0.001mol) of compound II-2 and 0.19g (0.001mol) of compound III-3 in 5ml of acetic acid, add 1ml of concentrated sulfuric acid, heat to 100°C, and react for 2h. After cooling, add 2 milliliters of perchloric acid (mass percentage concentration is 70%), then slowly add distilled water dropwise, precipitate solid, filter, vacuum dry, column chromatography purification and separation give 0.13g compound I-3, yield 27.7% . ESI MS: m / z, 369.2. lambda ab. max / nm=625nm,λ em max / nm=689nm,Ф f = 0.68.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com