Novel near-infrared high-quantum-yield dye as well as preparation and application thereof
A near-infrared, high-quantum technology, applied in the direction of benzoxanthene dyes, analytical materials, luminescent materials, etc., can solve problems such as long emission and difficulty in fluorescent quantum yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
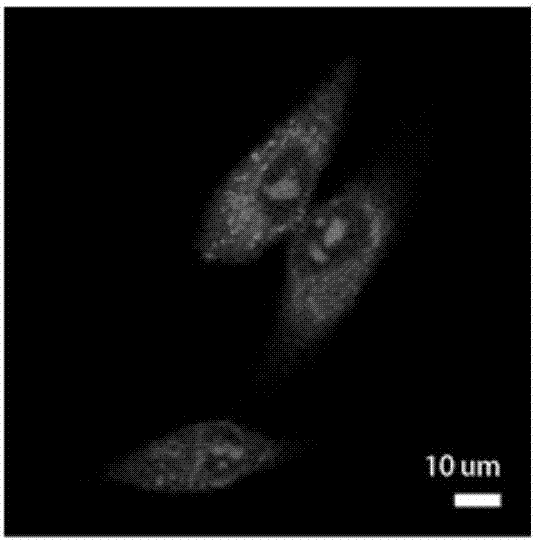
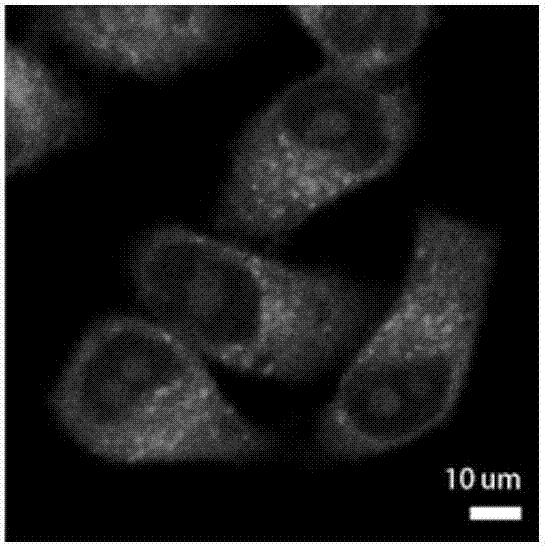
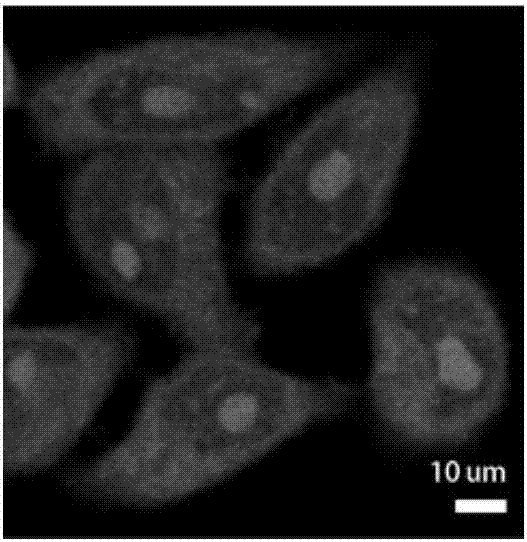
Image
Examples
Embodiment 1
[0103]
[0104] 0.193 g (0.001 mol) of compound II-1 and 0.161 g (0.001 mol) of compound III-1 were added to 5 ml of concentrated sulfuric acid, and then heated and stirred at 100° C. for 2 h. After cooling, the reaction solution was slowly poured into 200 ml of ice water, then 1 ml of perchloric acid (70%) was added dropwise under stirring, and a large amount of distilled water was added, and solids were precipitated after standing, filtered, and vacuum-dried , purified by column chromatography to obtain 0.343g of compound I-1, with a yield of 82%. ESI MS: theoretical calculation m / z: 319.18, actual test m / z: 319.20. lambda abs. max / nm=615nm,λ em max / nm=638nm,Ф f = 0.80.
[0105] 0.418g (0.001mol) of compound I-1, 0.202g (0.001mol) of di-tert-butyl dicarbonate and 0.101g (0.001mol) of triethylamine were added to 10 ml of anhydrous dichloromethane respectively, After stirring for 24 hours at room temperature, the solvent was removed under vacuum, dried and purified...
Embodiment 2
[0108]
[0109] Compound III-11 was prepared from commercial compound III-1 and ethyl iodide according to literature (Org. Lett., 2011, 13, 6488-6491), with a yield of 50%.
[0110] 0.217 g (0.001 mol) of compound II-2 and 0.189 g (0.001 mol) of compound III-2 were added to 5 ml of concentrated sulfuric acid, and then heated and stirred at 100° C. for 2 h. After cooling, the reaction solution was slowly poured into 200 ml of ice water, then 1 ml of perchloric acid (70%) was added dropwise under stirring, and a large amount of distilled water was added, and solids were precipitated after standing, filtered, and vacuum-dried , purified by column chromatography to obtain 0.399 g of compound I-4, with a yield of 85%. ESI MS: Theoretical calculation m / z: 371.21, actual test m / z: 371.24. lambda abs. max / nm=635nm,λ em max / nm=658nm,Ф f = 0.73.
[0111] Dissolve 0.470 g (0.001 mmol) of compound I-4 in 5 ml of anhydrous THF, and add dropwise to 3 ml of CH containing 1 mmol u...
Embodiment 3
[0113]
[0114]Compound II-3 was prepared from 7-hydroxy-1-methyl-1,2,3,4-tetrahydroquinoline according to the literature (J.Am.Chem.Soc., 2007,129,9986-9998), the yield 76%. Compound III-3 was prepared from commercial compound III-1 and methyl iodide according to literature (Org. Lett., 2011, 13, 6488-6491), with a yield of 42%.
[0115] 0.191 g (0.001 mol) of compound II-3 and 0.189 g (0.001 mol) of compound III-3 were added to 5 ml of concentrated sulfuric acid, and then heated and stirred at 100° C. for 2 h. After cooling, the reaction solution was slowly poured into 200 ml of ice water, then 1 ml of perchloric acid (70%) was added dropwise under stirring, and a large amount of distilled water was added, and solids were precipitated after standing, filtered, and vacuum-dried , purified by column chromatography to obtain 0.391 g of compound I-6, with a yield of 88%. ESI MS: Theoretical calculation m / z: 345.20, actual test m / z: 345.26. lambda abs. max / nm=633nm,λ em...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com