Preparation method of dobutamine hydrochloride intermediate compound
A technology for dobutamine hydrochloride and compound, applied in the field of medicinal chemistry, can solve the problems of high risk factor, low flash point of ether, high toxicity of benzene, etc., so as to reduce operational safety hazards, improve purity, yield, and safety risks low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
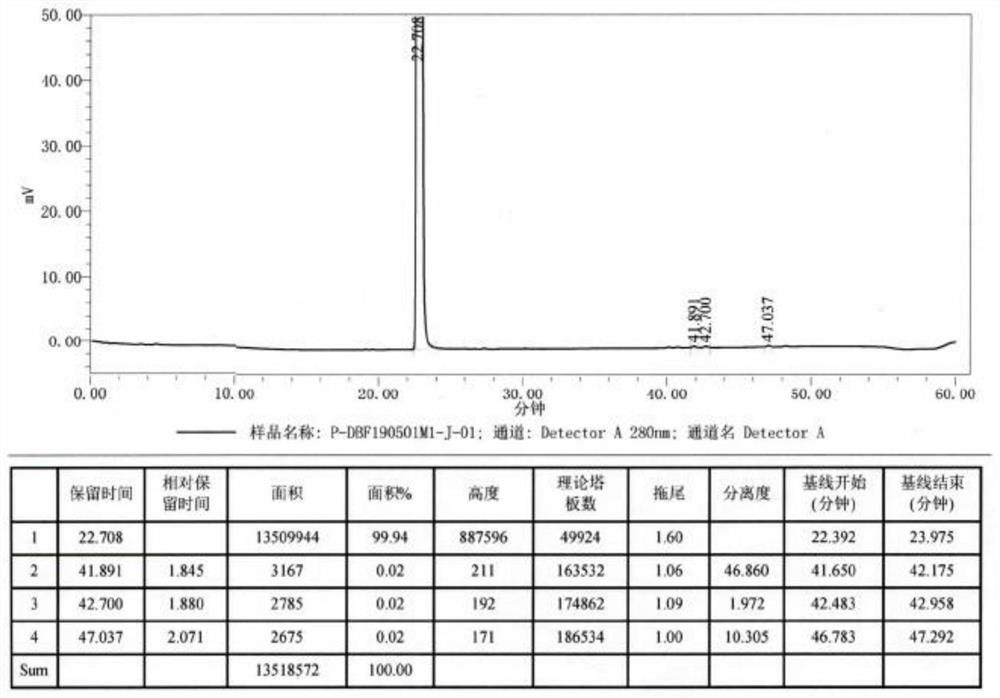
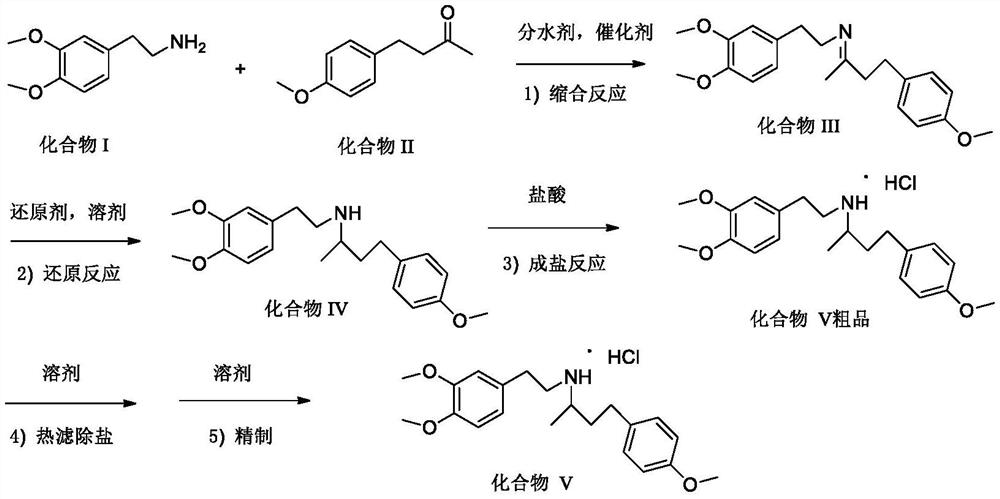
[0030] A preparation method of dobutamine hydrochloride intermediate (compound V),
[0031]
[0032] 1. Add 203g of 3,4-dimethoxyphenethylamine, 210g of 4-(4-methoxyphenyl)-2-butanone, 0.7g of acetic acid, and 1.2L of cyclohexane into a 3L reactor, and stir to raise the temperature 80-85°C to reflux, reflux for water separation reaction, heat preservation and reflux reaction for 6 hours, and no more water will come out at this time;
[0033] 2. Evaporate cyclohexane under normal pressure, recover it, and then cool down to room temperature, add 2.0L of absolute ethanol, 30.2g of potassium borohydride, stir and raise the temperature to 60-70°C, keep stirring and react for 2-3h, Stop heat preservation and cool down to below 40°C;
[0034] 3. Under the condition of stirring, add 0.2L of concentrated hydrochloric acid dropwise, cool down to 20-30°C, stir and keep warm for crystallization for 2-3h, filter, and dry the filter cake in a blast oven at 50-60°C to obtain 362g of filt...
Embodiment 2
[0038] A preparation method of dobutamine hydrochloride intermediate (compound V),
[0039]
[0040]1. Add 223g of 3,4-dimethoxyphenethylamine, 200g of 4-(4-methoxyphenyl)-2-butanone, 6.7g of acetic acid, and 1.2L of cyclohexane into a 3L reactor, and stir to raise the temperature 80-85°C to reflux, reflux for water separation reaction, heat preservation and reflux reaction for 6 hours, and no more water will come out at this time;
[0041] 2. Evaporate the cyclohexane under normal pressure, and then cool down to room temperature, add 2.0L of absolute ethanol, 30.2g of potassium borohydride, stir and react at room temperature for 10h, stop the heat preservation, and cool down to room temperature;
[0042] 3. Under the condition of stirring, add 0.2L of concentrated hydrochloric acid dropwise, cool down to 20-30°C, keep warm, stir and crystallize for 3 hours, filter, and dry the filter cake in a blast oven at 50-60°C to obtain 353g of filter cake;
[0043] 4. Add the above-...
Embodiment 3
[0046] A preparation method of dobutamine hydrochloride intermediate (compound V),
[0047]
[0048] 1. Add 203g of 3,4-dimethoxyphenethylamine, 260g of 4-(4-methoxyphenyl)-2-butanone, 0.7g of acetic acid, and 1.5L of cyclohexane into a 3L reactor, and stir to raise the temperature 80-85°C to reflux, reflux for water separation reaction, heat preservation and reflux reaction for 6 hours, and no more water will come out at this time;
[0049] 2. Evaporate the cyclohexane under normal pressure, and then cool down to room temperature, add 3.0L of isopropanol, add 21.2g of sodium borohydride in portions, stir and heat up to 60-70°C, keep warm for 2 hours, stop keeping warm , cooled to room temperature;
[0050] 3. Under the condition of stirring, add 0.2L of concentrated hydrochloric acid dropwise, cool down to 20-30°C, stir and keep warm for crystallization for 3 hours, filter, and dry the filter cake in a blast oven at 50-60°C to obtain 349g of filter cake;
[0051] 4. Add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com