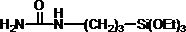A class of silane coupling agent with ureido and imide structures, preparation method and application thereof
A silane coupling agent, alkyl technology, applied in the field of organosilicon compounds, can solve the problems of high price of phenylethynyl phthalic anhydride, unsuitable for large-scale industrial use and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
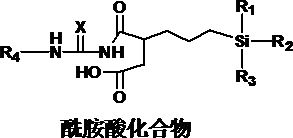
[0055] The preparation method of silane coupling agent of the present invention is as follows:
[0056] 1) The compound represented by formula (2) and the compound represented by formula (3) undergo condensation reaction;
[0057] 2) Imidization reaction is carried out after the condensation reaction to obtain the silane coupling agent represented by formula (1).
[0058]
[0059]
[0060] X, R in formula (2) and (3) 1 , R 2 , R 3 , R 4 The definitions are the same as those mentioned above.
[0061] Further, the condensation reaction is carried out in an aprotic polar solvent, and the effect of each aprotic polar solvent is equivalent. Considering the convenience of cost and acquisition method, preferably, the aprotic polar solvent is selected from N -Methylpyrrolidone, N,N -dimethylformamide, N,N -at least one of dimethylacetamide, dimethylsulfoxide and gamma-butyrolactone, preferably N -Methylpyrrolidone or / and N,N - Dimethylacetamide.
[0062] Further, the ...
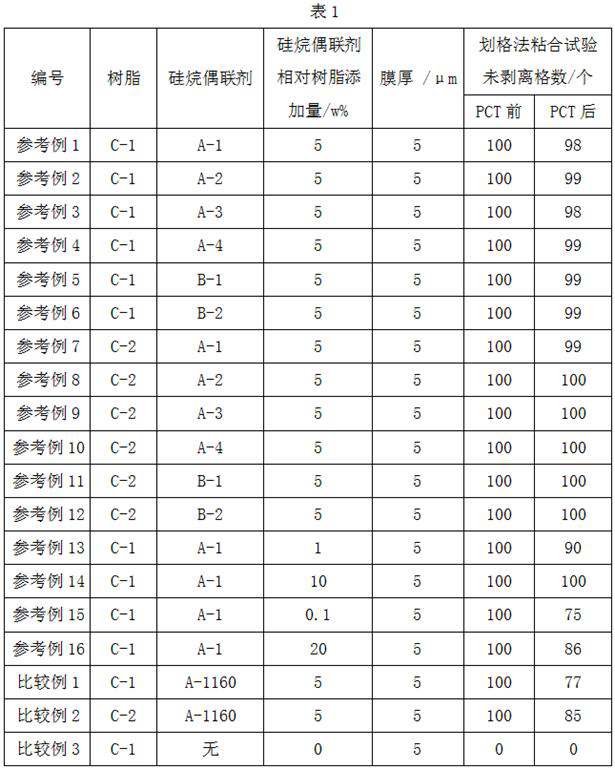
Embodiment 1
[0070] Add 26.2 g (0.1 mol, X-12-967C, Shin-Etsu Chemical) of 3-(trimethoxysilyl)propyl succinic anhydride to a 500 mL three-necked flask equipped with a stirrer and a thermometer, and add the solvent N,N- Dimethylacetamide 150 mL, start stirring. At the same time, 7.4 g (0.1 mol, Tokyo Chemical Industry Co., Ltd.) of methylurea was weighed and dissolved in 100 mL N,N-in dimethylacetamide. Place the above-mentioned 500 mL three-necked flask in an ice-water bath, slowly add methylurea solution dropwise while stirring, and control the temperature of the reaction material below 10°C. After the dropwise addition was completed, the ice-water bath was removed to return to room temperature, and the reaction was continued at this temperature for 20 hr. After the reaction was completed, 15.8 g (0.2 mol) of pyridine was added to the reaction system, and after stirring evenly, 20.4 g (0.2 mol) of acetic anhydride was slowly added, and the reaction was carried out at room temperature ...
Embodiment 2
[0074] Add 26.2 g (0.1 mol, X-12-967C, Shin-Etsu Chemical) of 3-(trimethoxysilyl)propyl succinic anhydride to a 500 mL three-neck flask equipped with a stirrer and a thermometer, add the solvent N,N- Dimethylacetamide 150 mL, start stirring. At the same time, weigh 8.8 g (0.1 mol, Tokyo Chemical Industry Co., Ltd.) of ethyl urea and dissolve it in 100 mL N,N- in dimethylacetamide. Place the above-mentioned 500 mL three-neck flask in an ice-water bath, slowly add ethyl urea solution dropwise while stirring, and control the temperature of the reaction material below 10°C. After the dropwise addition was completed, the ice-water bath was removed to return to room temperature, and the reaction was continued at this temperature for 20 hr. After the reaction was completed, 15.8 g (0.2 mol) of pyridine was added to the reaction system, and after stirring evenly, 20.4 g (0.2 mol) of acetic anhydride was slowly added, and the reaction was carried out at room temperature for 20 hr. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com