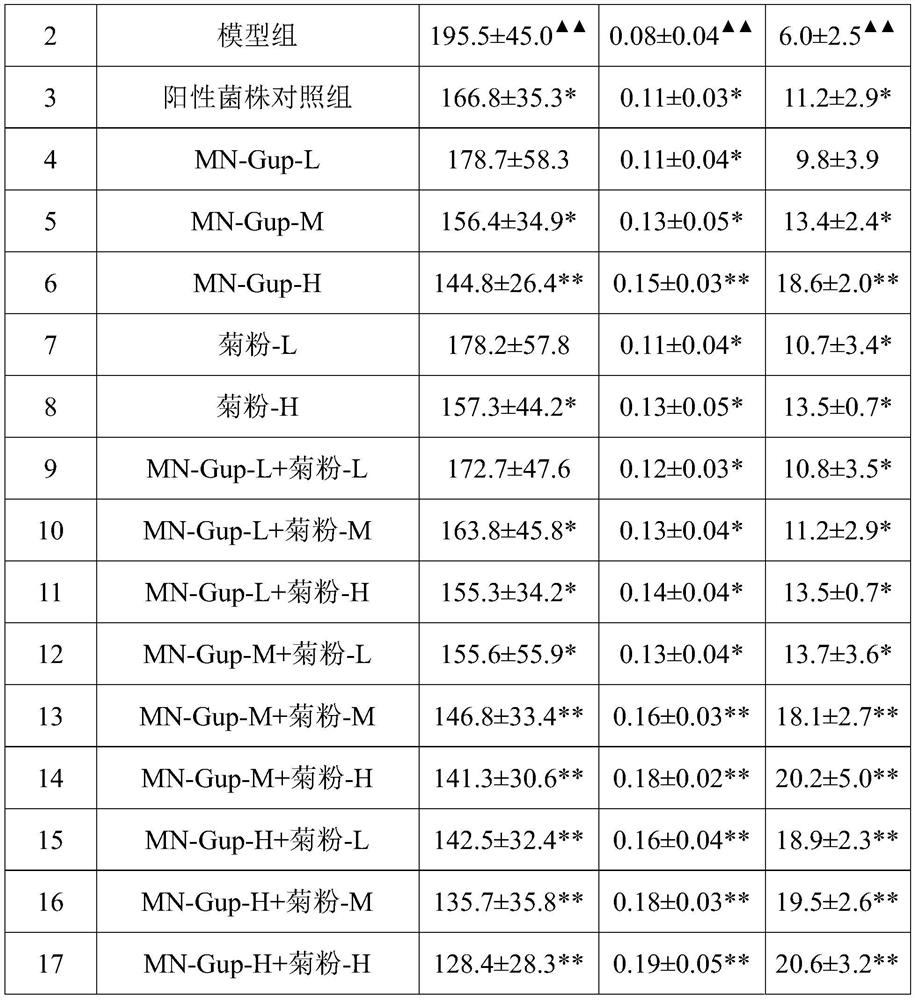
Bifidobacterium animalis with bowel relaxing function as well as application and preparation of bifidobacterium animalis
A technology for animal bifidobacteria and bowel laxatives, applied in bifidobacteria, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc. The high cost of yogurt can achieve the effect of good laxative function, enhanced oxygen tolerance and broad application prospects.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] This example provides a Bifidobacterium animalis MN-Gup, the preservation number of which is CGMCC No.15578.
[0024]In the present invention, Bifidobacterium animalis MN-Gup is obtained by strain isolation and aerospace mutagenesis of bacterial strains. Specifically, (1) the separation of bacterial strains is as follows: collecting intestinal contents of healthy and long-lived elderly people in Bama, Guangxi, and That is feces, the intestinal feces samples of the healthy and long-lived elderly in Bama, Guangxi were gradiently diluted with diluent, wherein, the preparation method of diluent: sodium dihydrogen phosphate 4.5g, disodium hydrogen phosphate 6.0g, L-cysteine 0.5 g, agar 0.5g, Tween 80 0.5ml, distilled water 1000ml, heat to dissolve, adjust the pH to 7.4-7.6, sterilize at 121°C for 15min, and use NPNL-X-Gal selective medium (containing neomycin sulfate , Paromomycin Sulfate, Sodium Propionate, Lithium Chloride) anaerobically isolate Bifidobacteria (Yang Haiy...
Embodiment 2
[0026] This embodiment provides a preparation, including Bifidobacterium animalis MN-Gup and inulin. Based on every gram of the preparation, the content of Bifidobacterium animalis MN-Gup is 4×10 6 CFU / g, the content of described inulin is 10mg / g;
[0027] The preparation method of above-mentioned preparation, comprises the steps:
[0028] (1) Preparation of bacterial suspension: culture the Bifidobacterium animalis MN-Gup strain with MRS liquid culture at 37°C for 12 hours, centrifuge at 4500r / min for 15min, and resuspend the bacterial pellet in normal saline , formulated as 8 x 10 6 CFU / g of bacterial suspension;
[0029] (2) Configuration of inulin: accurately weigh 0.2 g of inulin and dissolve it in 10 g of physiological saline (20 mg / g) to obtain inulin solution;
[0030] (3) Take the equal mass of the bacterial suspension and mix the inulin solution to obtain the preparation.
Embodiment 3
[0032] This embodiment provides a preparation, including Bifidobacterium animalis MN-Gup and inulin. Based on every gram of the preparation, the content of Bifidobacterium animalis MN-Gup is 4×10 6 CFU / g, the content of described inulin is 25mg / g.
[0033] The preparation method of above-mentioned preparation, comprises the steps:
[0034] (1) Preparation of bacterial suspension: culture the Bifidobacterium animalis MN-Gup strain with MRS liquid culture at 37°C for 12 hours, centrifuge at 4500r / min for 15min, and resuspend the bacterial pellet in normal saline , formulated as 8 x 10 6 CFU / g of bacterial suspension;
[0035] (2) Configuration of inulin: accurately weigh 0.5 g of inulin and dissolve it in 10 g of physiological saline (50 mg / g), to obtain inulin solution;
[0036] (3) Take the equal mass of the bacterial suspension and mix the inulin solution to obtain the preparation.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com