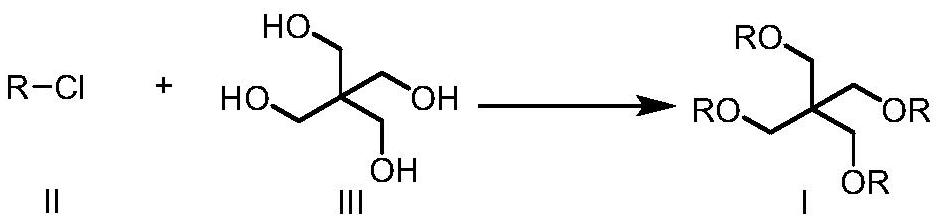
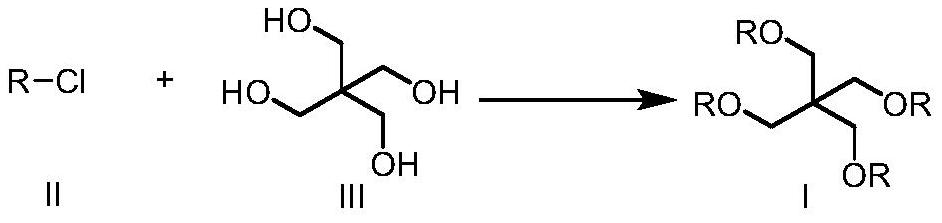
Preparation method of pentaerythritol tetraisostearate
A technology of isostearate and pentaerythritol tetra, applied in the field of preparation of pentaerythritol tetraisostearate, can solve the problems of high reaction temperature, dark color of reaction solution, difficult removal of acid, etc., and achieves reduction of reaction temperature and reaction conditions. Mild, short response time effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthesis of embodiment 1 isostearyl chloride
[0026] To a solution of isostearic acid (100 g, 352 mmol) in dichloromethane (100 mL) was added dropwise thionyl chloride (46.1 g, 387 mmol) at 10-20°C under nitrogen atmosphere. After dropping, the temperature was raised to 35-45°C and stirred for 2 hours. TLC showed that the reaction was complete. After concentration, 107.6 g of light yellow liquid was obtained, which was set aside.
Embodiment 2
[0027] The synthesis of embodiment 2 isostearyl chloride
[0028] To a solution of isostearic acid (100 g, 352 mmol) in dichloromethane (100 mL) was added dropwise oxalyl chloride (46.9 g, 370 mmol) at 10-20 °C under nitrogen atmosphere. After dropping, the temperature was raised to 35-45°C and stirred for 2 hours. TLC showed that the reaction was complete. After concentration, 106.8 g of nearly colorless liquid was obtained, which was set aside.
Embodiment 3
[0029] The synthesis of embodiment 3 isostearyl chloride
[0030] To a solution of isostearic acid (100 g, 352 mmol) in dichloromethane / DMF (100 mL / 1 mL) was added triphosgene (41.8 g, 141 mmol) at 10-20 °C under nitrogen atmosphere. After the addition was complete, the temperature was raised to 35-45°C and stirred for 2 hours. TLC showed that the reaction was complete. After concentration, 108.2 g of nearly colorless liquid was obtained, which was set aside.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com