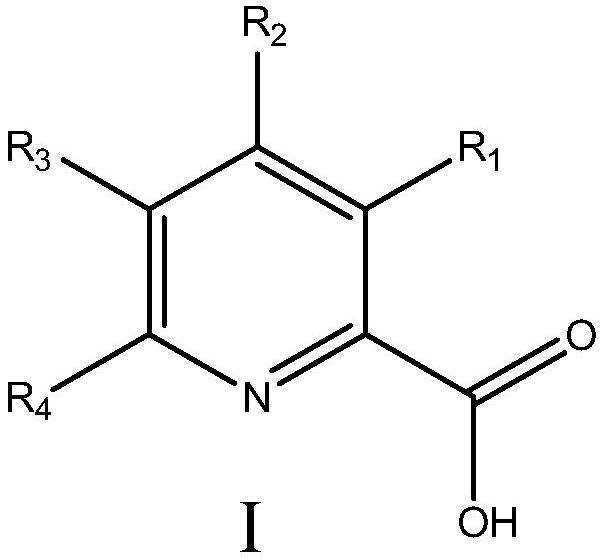
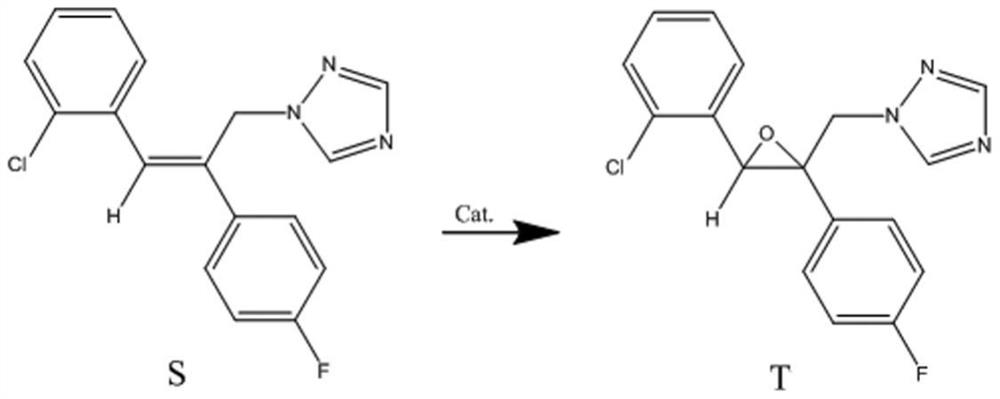
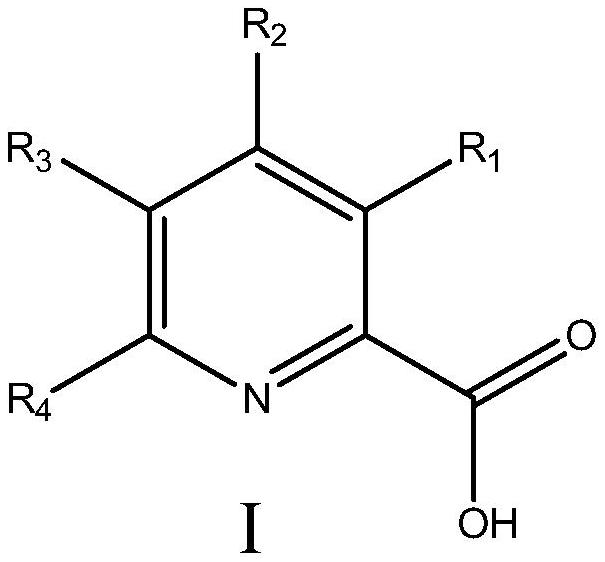
Manganese catalyst and application of manganese catalyst in catalyzing epoxidation of triazole alkene to prepare epoxiconazole
A technology for catalyzing triazoles and manganese catalysts, applied in the field of pesticides, can solve problems such as hidden safety hazards, unfriendly environment, increase post-processing steps, etc., and achieve the effects of reducing environmental pressure, low system cost, and simple recovery
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 7.4mg (0.03mmol) of manganese acetate tetrahydrate into a 100mL one-necked flask at room temperature, add 5mL of acetonitrile, and 22.2mg (0.18mmol) of picolinic acid, and start stirring. Under stirring, add 3.14g (10.0mmol, content 99.68%) of recrystallized triazolene, 0.43g (5.0mmol) of distilled 2,3-butanedione, 24.6mg of sodium acetate dispersed in 15mL of acetonitrile solution, and continue stirring . Cool the flask in an ice bath and add 30% hydrogen peroxide dropwise below 2°C, add 3.40g hydrogen peroxide within 5.0hrs, during which the dropping rate should be controlled so that the temperature of the reaction solution is not higher than 5°C. After the hydrogen peroxide was added, the mixture was stirred for 1.0 hr in a cold bath. Remove the ice / water bath, and stir at room temperature for 2.0 hrs, concentrate under reduced pressure to obtain the target epoxiconazole, the material conversion rate is 99%, and the epoxiconazole yield is 65% (HPLC detection)
Embodiment 2
[0031] Add 7.4mg (0.03mmol) of manganese acetate tetrahydrate into a 100mL one-necked flask at room temperature, add 20mL of acetonitrile, and 22.2mg (0.18mmol) of picolinic acid, and start stirring. Under stirring, add 3.14g (10.0mmol, content 99.68%) of recrystallized triazolene, and 0.43g (5.0mmol) of distilled 2,3-butanedione, and stir at room temperature for 15min, then add 24.6mg of sodium acetate , continue stirring. Cool the flask in an ice bath and add 30% hydrogen peroxide dropwise at a temperature below 2°C. Add 3.40g of hydrogen peroxide within 5.0hrs, during which the rate of addition should be controlled so that the temperature of the reaction solution is not higher than 5°C. After the hydrogen peroxide was added, the mixture was stirred for 1.0 hr in a cold bath. Remove the ice / water bath and stir at room temperature for 2.0 hrs. The material conversion rate is 99%, and the yield of epoxiconazole is 66% (HPLC detection).
Embodiment 3
[0032] Example 3: Add 6.9 mg (0.028 mmol) of manganese acetate tetrahydrate into a 100 mL one-necked flask at room temperature, add 20 mL of acetonitrile, and 20.9 mg (0.17 mmol) of picolinic acid, and start stirring. Under stirring, add 3.14g (10.0mmol, content 99.68%) of recrystallized triazolene, 0.43g (5.0mmol) of distilled 2,3-butanedione, and stir at room temperature for 15min, then add 23.0mg of sodium acetate, Continue to stir. Cool the flask in an ice bath and add 30% hydrogen peroxide dropwise below 2°C, add 3.40g hydrogen peroxide within 5.0hrs, during which the dropping rate should be controlled so that the temperature of the reaction solution is not higher than 5°C. After the hydrogen peroxide was added, the mixture was stirred for 1.0 hr in a cold bath. Remove the ice / water bath, stir at room temperature for 2.0 hrs, and concentrate under reduced pressure to obtain the target epoxiconazole. The material conversion rate was 98%, and the epoxiconazole yield was 6...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com