Streptococcus pneumoniae freeze-drying protective agent
A freeze-drying protective agent, a technology for Streptococcus pneumoniae, which is applied in the preservation of microorganisms, microorganisms, biochemical equipment and methods, etc., can solve the problems of slow growth in the fermentation process, mutation or degradation of Streptococcus pneumoniae species, loss of immunogenicity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
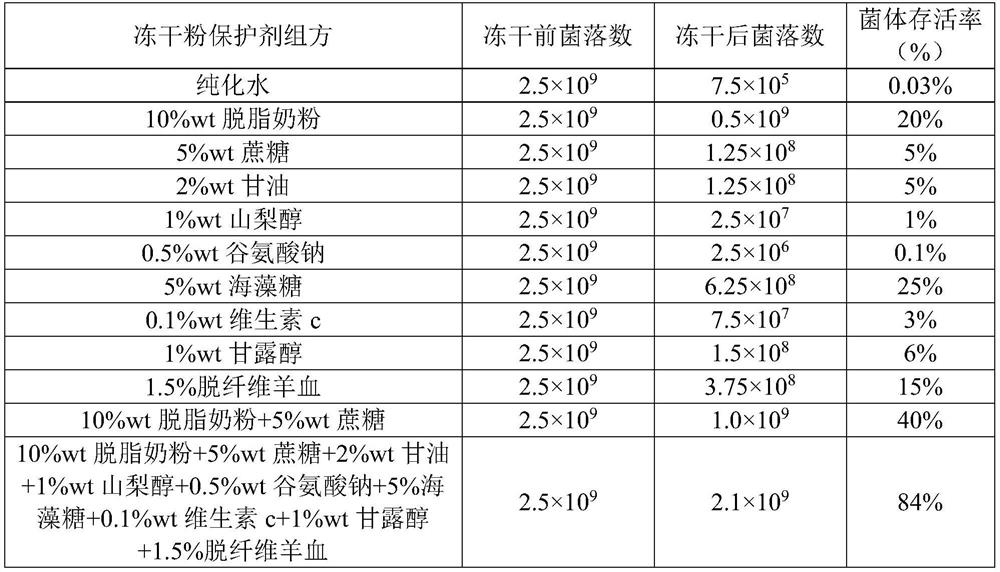
[0028] Streak pneumoniae type 1 bacteria on the blood plate, culture overnight at 37°C and 5% carbon dioxide environment, inoculate the grown single colony into 30ml THB medium and culture for 5 hours, then divide into 2ml sterile EP tubes , 1ml per tube. Put it into a centrifuge at 12000rpm, centrifuge for 5min, remove the supernatant, and obtain the thalline that needs to be freeze-dried. After formulating the lyophilized protective agent according to the lyophilized protective agent formula in the table below, take 1ml of the protective agent and resuspend the bacteria in the EP tube to make a bacterial suspension, and resuspend 2 EP tubes for each lyophilized protective agent . Before freeze-drying, take one EP tube resuspended with various freeze-dried powder protective agents, and spread the plate after gradient dilution to calculate the number of colonies; resuspend each freeze-dried powder with 1ml PBS, spread gradient dilution Count the number of colonies on the pla...
Embodiment 2
[0033] Preparation of 100ml lyoprotectant: Weigh 1g of sucrose, 2g of glycerin, 1g of sorbitol, 10g of skim milk powder, 0.5g of sodium glutamate, 5g of trehalose, 0.1g of vitamin C, 1g of mannitol, 1.5g of defibrated sheep blood, After dissolving with water for injection, dilute to 100ml, and then use a 0.22μm filter to filter and sterilize.
[0034] Streptococcus pneumoniae type 1 was rejuvenated on the Columbia blood plate, and the bacterial lawn was picked and put into the freeze-dried protective agent configured above to make a bacterial suspension (bacterial density of about 10 9 ~10 10 cfu), and then divided into ampoules, each ampoule 0.3ml bacterial solution.
[0035] Take out an aliquoted ampoule, dilute the bacterial solution in an appropriate gradient, spread it on a plate, put it in a carbon dioxide incubator at 37°C for overnight culture, and count the bacteria after they grow out.
[0036] Put other ampoules in a freeze dryer to freeze-dry, take a freeze-dried...
Embodiment 3
[0040] Preparation of 100ml lyoprotectant: Weigh 5g of sucrose, 2g of glycerin, 1g of sorbitol, 12g of skim milk powder, 0.5g of sodium glutamate, 5g of trehalose, 0.1g of vitamin C, 1g of mannitol, 1.5g of defibrated sheep blood, After dissolving with water for injection, dilute to 100ml, and then use a 0.22μm filter to filter and sterilize.
[0041]Streptococcus pneumoniae type 14 was rejuvenated on the Columbia blood plate, and the bacterial lawn was picked and put into the freeze-dried protective agent configured above to make a bacterial suspension (bacterial density of about 10 9 ~10 10 cfu), and then divided into ampoules, 0.3ml bacterial solution in each ampoule.
[0042] Take out an aliquoted ampoule, dilute the bacterial solution in an appropriate gradient, spread it on a plate, put it in a carbon dioxide incubator at 37°C for overnight culture, and count the bacteria after they grow out.
[0043] Put other ampoules into a freeze dryer to freeze-dry, take a freeze-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com