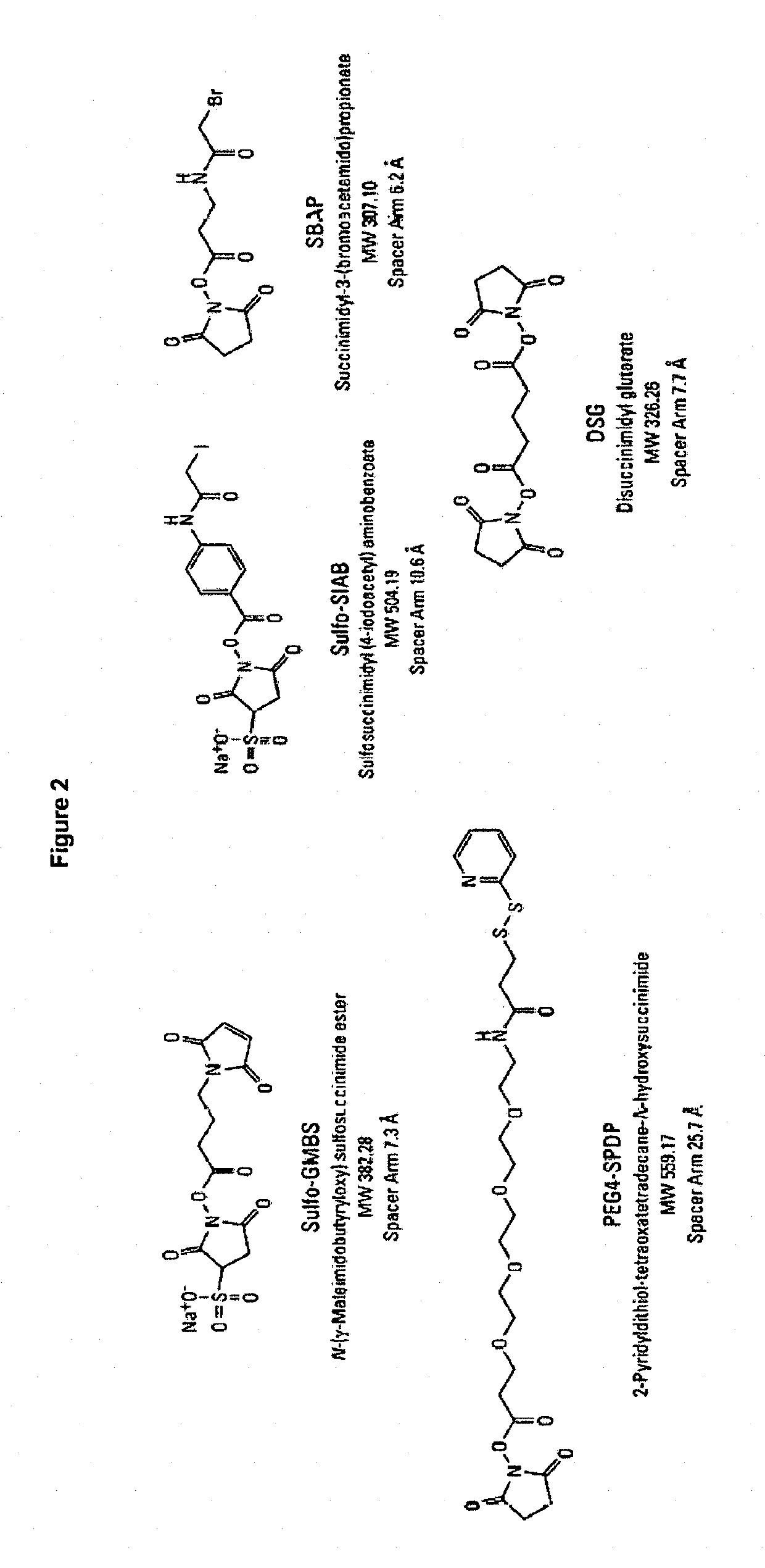
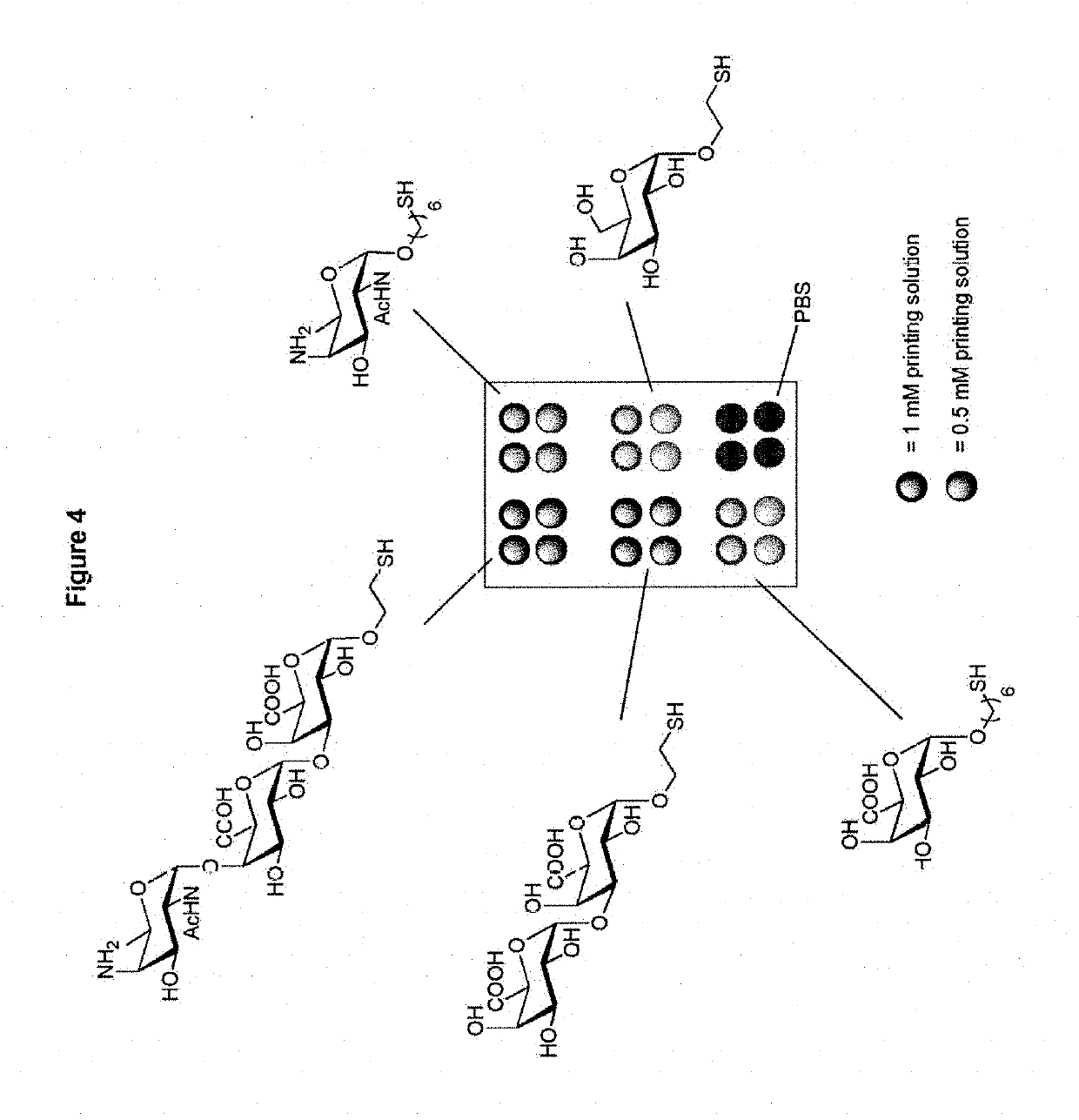
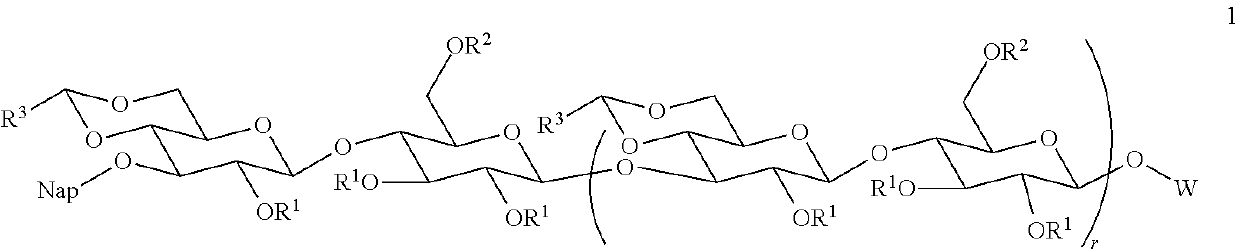
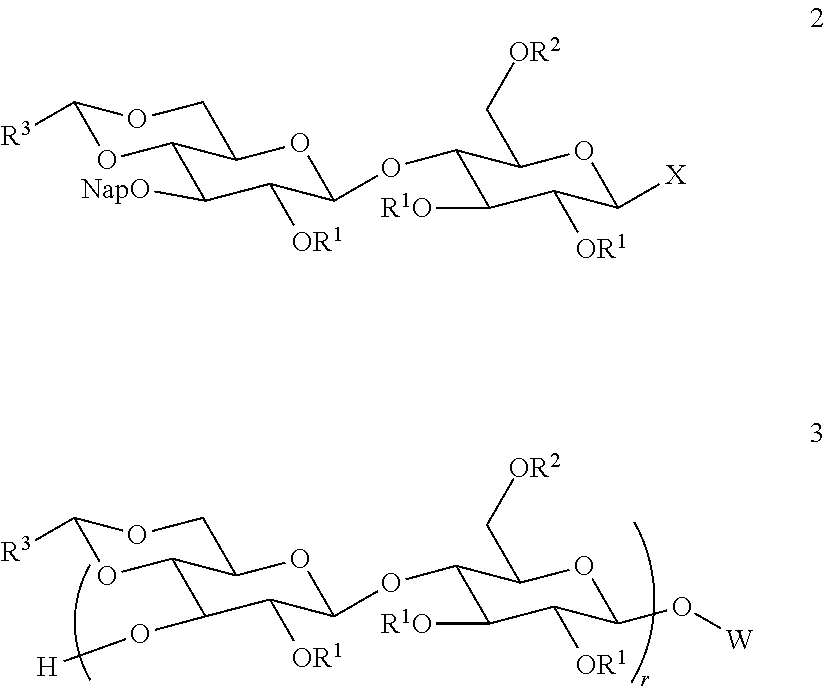
Patents
Literature
52 results about "Streptococcus pneumoniae pneumonia" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Streptococcus pneumonia is a type of highly contagious respiratory infection. It is caused by the bacteria Streptococcus pneumoniae, a widespread pathogen that can also cause sinusitis, ear infections, and other health complications.
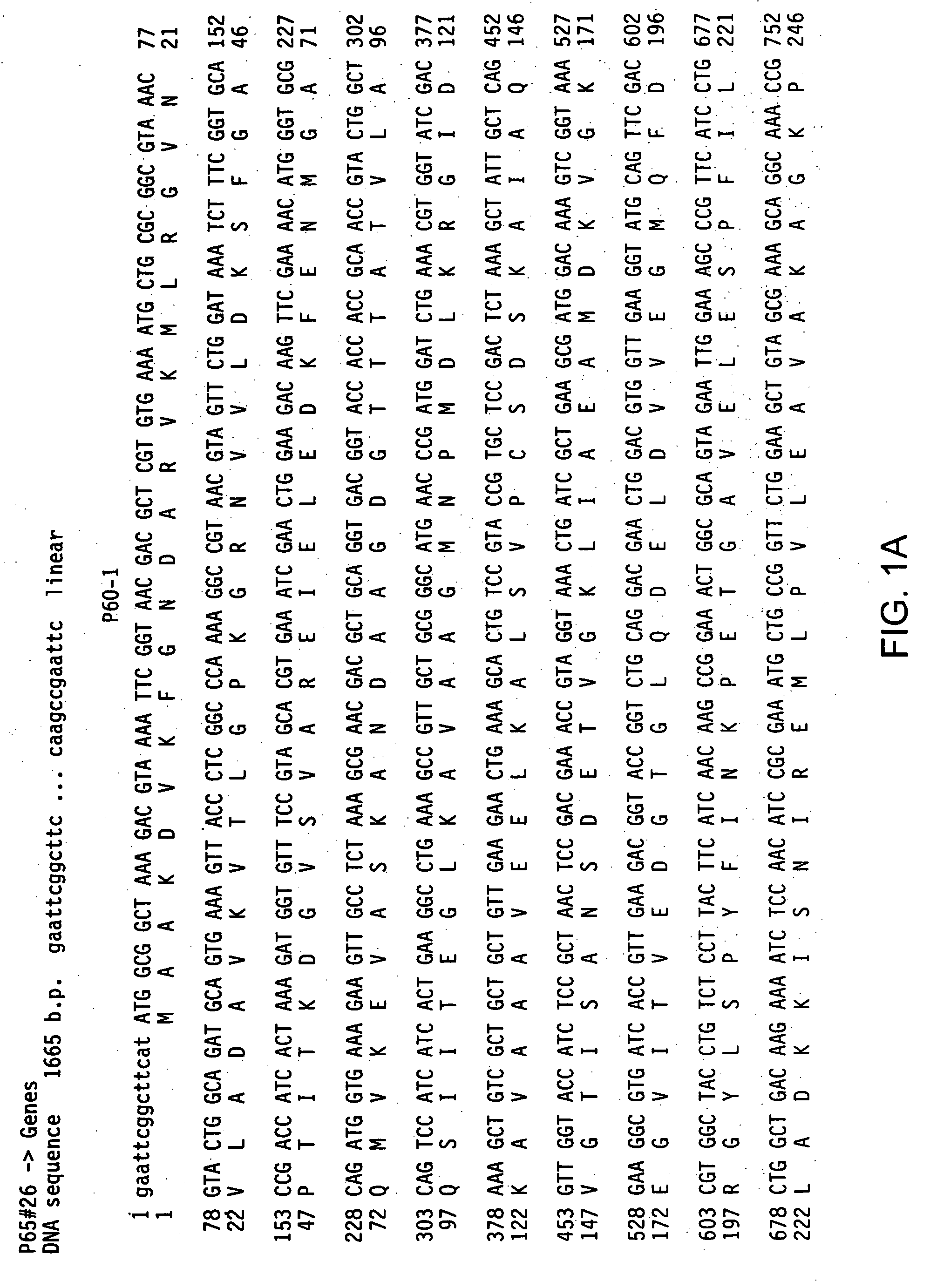
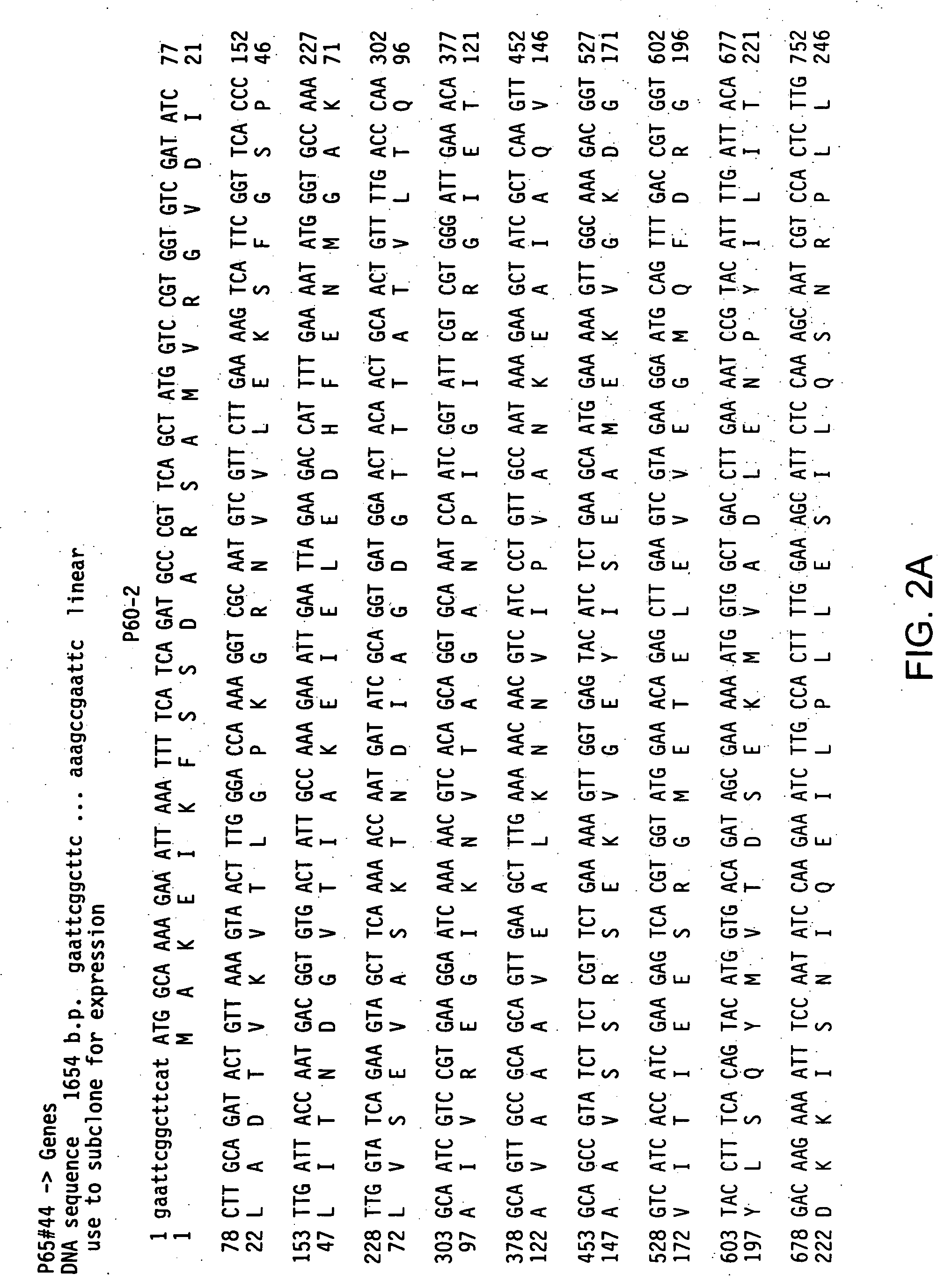
Streptococcus pneumoniae SP042 polynucleotides
The present invention relates to novel vaccines for the prevention or attenuation of infection by Streptococcus pneumoniae. The invention further relates to isolated nucleic acid molecules encoding antigenic polypeptides of Streptococcus pneumoniae. Antigenic polypeptides are also provided, as are vectors, host cells and recombinant methods for producing the same. The invention additionally relates to diagnostic methods for detecting Streptococcus nucleic acids, polypeptides and antibodies in a biological sample.
Owner:HUMAN GENOME SCI INC
Streptococcal heat shock proteins of the Hsp60 family
Methods and compositions comprising isolated nucleic acid molecules specific to Streptococcus pneumoniae and Streptococcus pyogenes, as well as vector constructs and isolated polypeptides specific to Streptococcus pneumoniae and Streptococcus pyogenes are provided. Such compositions and methods are useful for the diagnosis of Streptococcal infection and for generating an immune response to Streptococcal bacteria.
Owner:NVENTA BIOPHARMACEUTICALS CORP
Surface-located streptococcus pneumoniae polypeptides
InactiveCN101163499AAntibacterial agentsBacterial antigen ingredientsStreptococcus pneumoniaeStreptococcus mitis
The present invention relates to cell-surface-located polypeptides of Streptococcus pneumoniae and their use in immunisation against Streptococcal infection, in diagnosis of Streptococcus and in identification of compounds with anti-Streptococcus activity. In a further aspect, the invention relates to antibodies capable of recognising cell surface-located polypeptides of Streptococcus pneumoniae and uses thereof.
Owner:ACE BIOSCIENCES AS
Streptococcus pneumonia fusion protein and vaccine thereof
InactiveCN105968213AImprove protectionAntibacterial agentsBacterial antigen ingredientsSpecific iggStreptococcus mitis
The invention relates to the technical field of biology, in particular to streptococcus pneumonia fusion protein and a vaccine thereof. The fusion protein is an expression product formed by recombination of a streptococcus pneumonia virulence protein gene and a protein connector gene, and streptococcus pneumonia virulence protein is Ply or PspA or PsaA or PcpA or PhtD. After the vaccine of the streptococcus pneumonia fusion protein immunizes an animal through the nasal cavity, a high-potency serum specific IgG antibody can be induced, high-potency local mucosa specific IgA can be induced, and a good protective effect is achieved for nasal cavity toxin counteracting of different streptococcus pneumonia strains.
Owner:CHANGCHUN BCHT BIOTECH +1
Immunogenic composition
InactiveUS20140105927A1High activityBacterial antigen ingredientsCarrier-bound antigen/hapten ingredientsSerotypeBiochemistry
The present invention relates to an immunogenic composition comprising(i) at least one Streptococcus pneumoniae protein;(ii) a Streptococcus pneumoniae capsular saccharide derived from a strain of a targeted serotype of Streptococcus pneumoniae; for use in enhancing antibody-mediated opsonic activity against the targeted serotype of Streptococcus pneumoniae.
Owner:GLAXOSMITHKLINE BIOLOGICALS SA
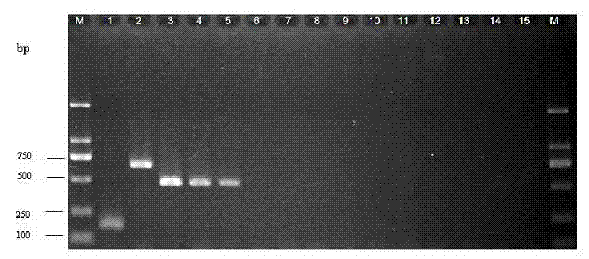
Multiple landing PCR(Polymerase Chain Reaction) detection kit and detection method for pathogenic bacteria of lower respiratory tract
InactiveCN102409103AHigh sensitivityMicrobiological testing/measurementMicroorganism based processesBovine serum albuminEpidemiologic survey
The invention designs specific primers for streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex, and establishes a multiple landing PCR(Polymerase Chain Reaction) detection kit and a detection method for simultaneously detecting the bacteria, adopting agarose gel electrophoresis for detecting PCR products. The kit comprises a 10*PCR buffer solution, MgCl2, dNTP, TaqDNA polymerase, BSA(Bovine Serum Albumin), positive control DNAs of streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex, a streptococcus pneumoniae primer, a haemophilus influenzae type b primer and a mycobacterium tuberculosis complex primer. The kit can simultaneously quickly detect streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex in a lower respiratory tract sample. The multiple landing PCR detection kit and detection method established by the invention are quick, simple, specific and sensitive, and can be used for rapid diagnosis and epidemiological investigation of infection of streptococcus pneumoniae, haemophilus influenzae type b and mycobacterium tuberculosis complex.
Owner:JIANGSU UNIV
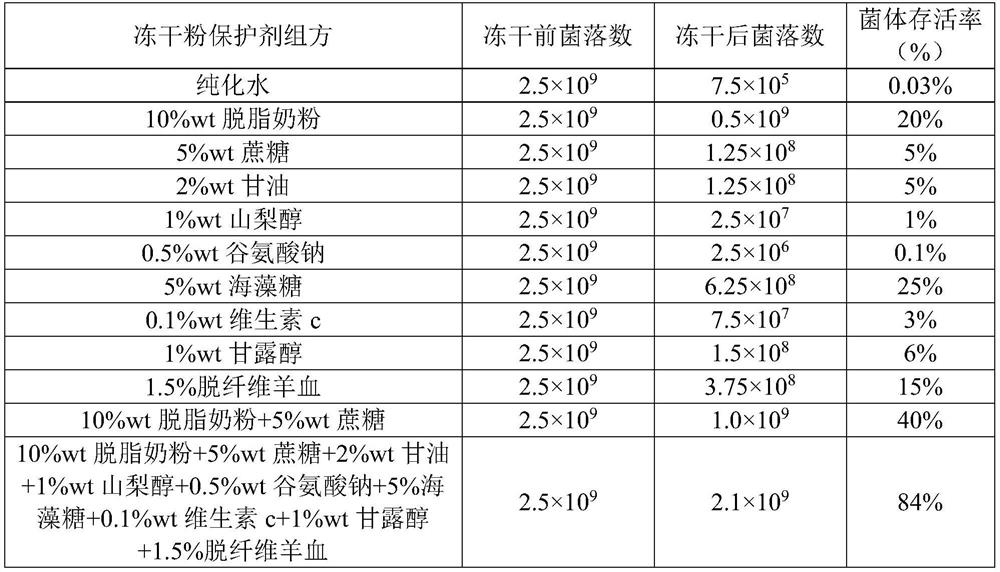
Streptococcus pneumoniae freeze-drying protective agent
ActiveCN111849781AStable traitsNo degradationMicroorganism based processesMicroorganism preservationBiotechnologySucrose
The invention discloses a streptococcus pneumoniae freeze-drying protective agent which comprises skim milk powder with the concentration of 1 wt%-20 wt% and cane sugar with the concentration of 1 wt%-10 wt%. The survival rate of thalli in a streptococcus pneumoniae freeze-drying tube prepared from the freeze-drying protective agent reaches 80% or above, the character is stable, and the freeze-drying protective agent is suitable for preparing strains for streptococcus pneumoniae vaccine production.
Owner:SHANGHAI RONGSHENG BIOLOGICAL PHARM CO LTD
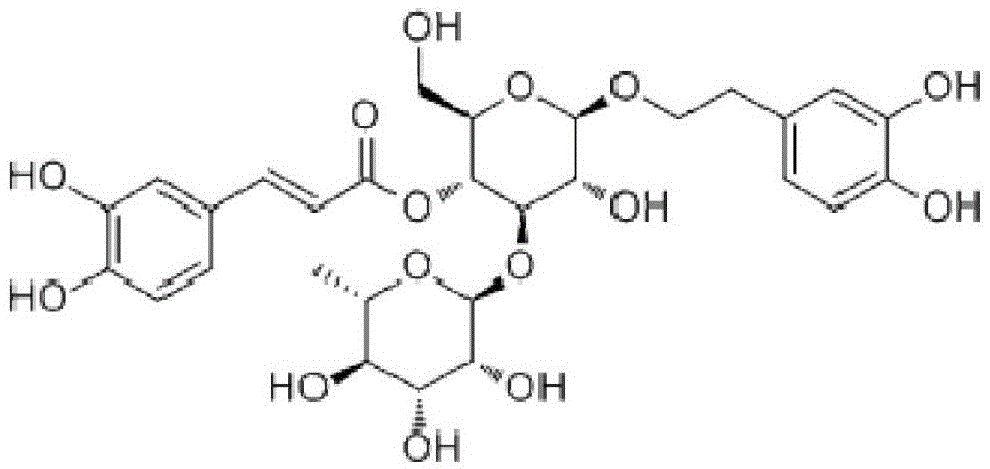
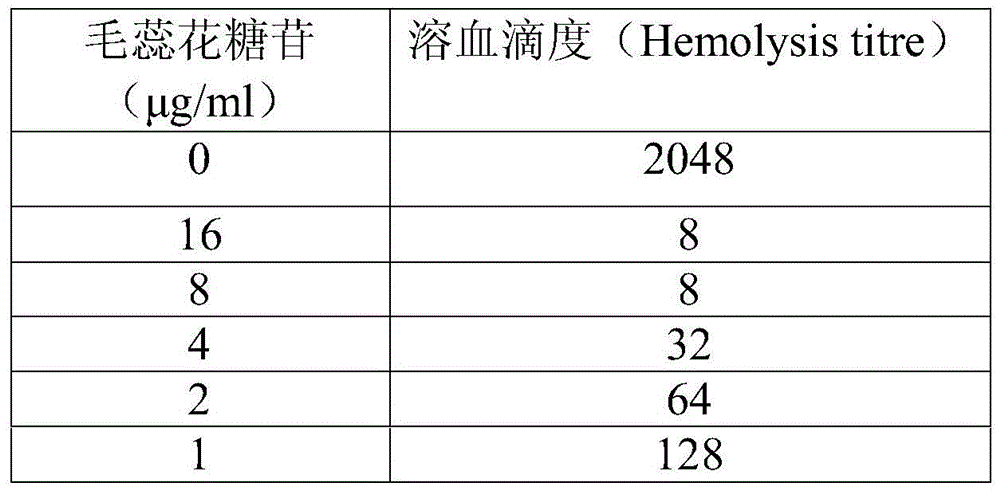
Application of verbascoside in preparation of pneumonia treatment drug
The invention relates to application of verbascoside in preparation of a pneumonia treatment drug. The treatment effect of the verbascoside to streptococcus pneumoniae infection is proved through sheep red blood cell hemolysis test, protection experiment of the human pulmonary epithelial cell (A549) damage and a mouse streptococcus pneumoniae pneumonia pneumonia model. Compared with antibiotic treatment, when the verbascoside is used for treatment, so that the pneumonia treatment drug has the advantages that no drug resistance exists and the curing rate is high.
Owner:JILIN UNIV
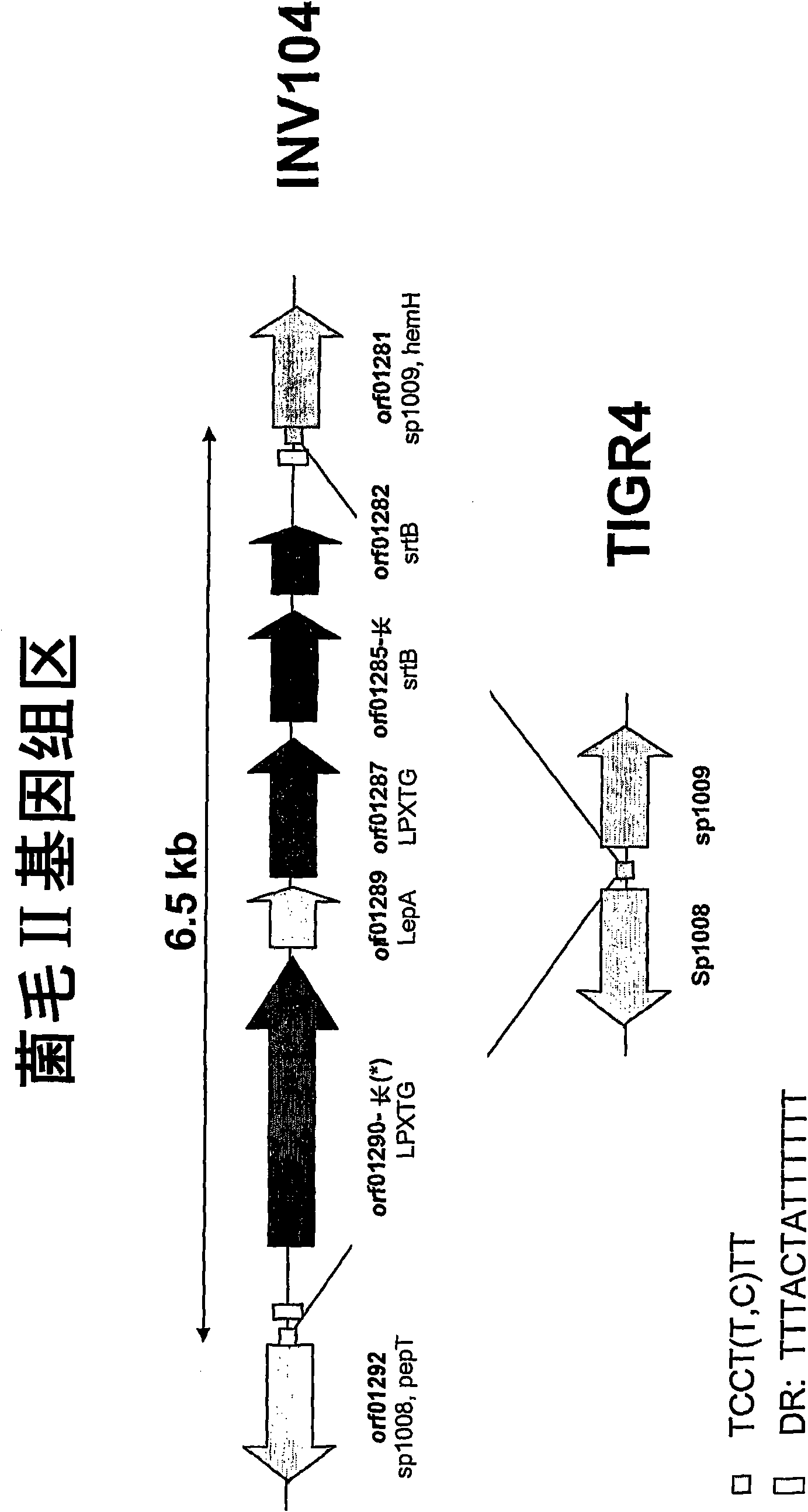
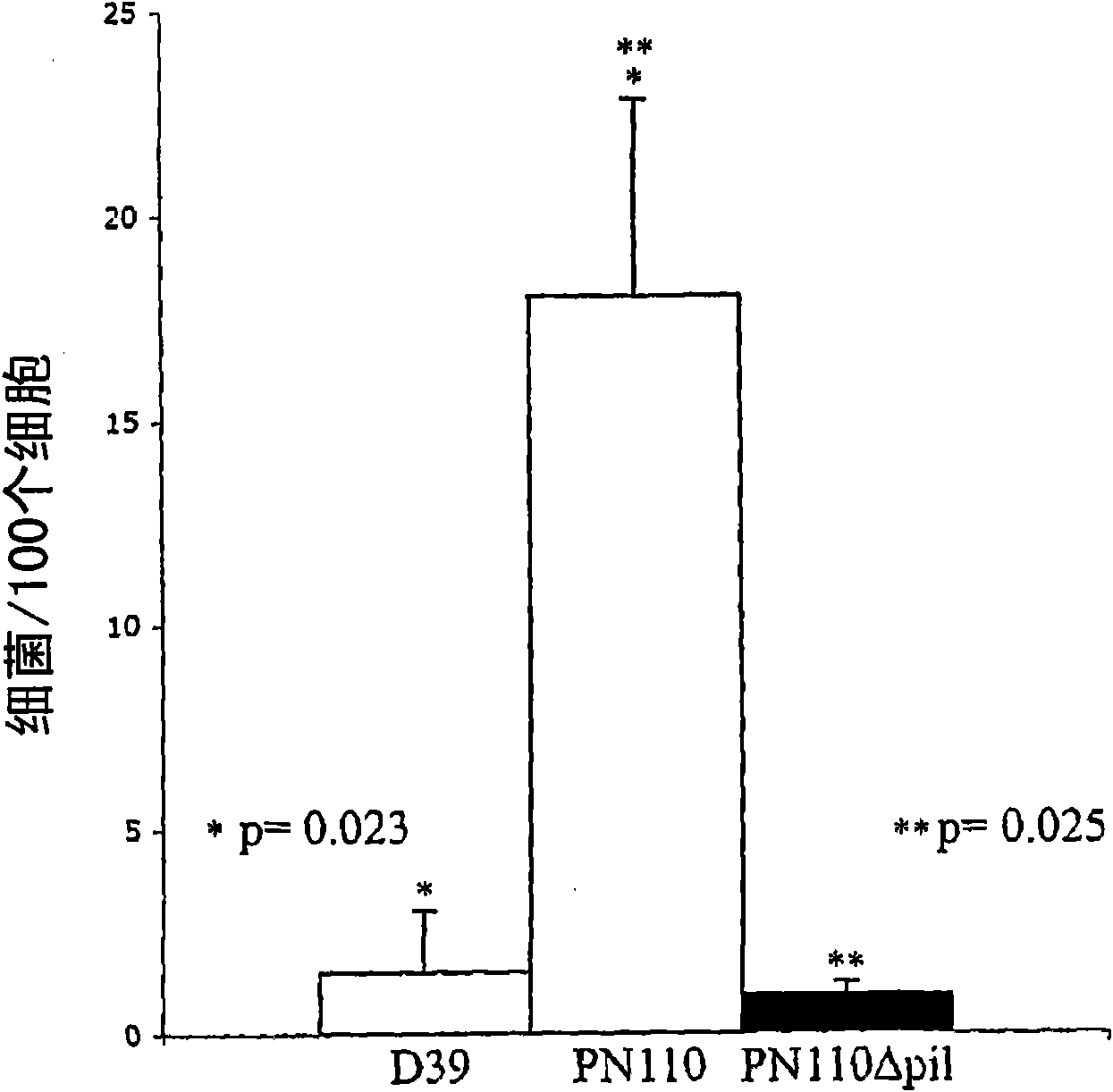
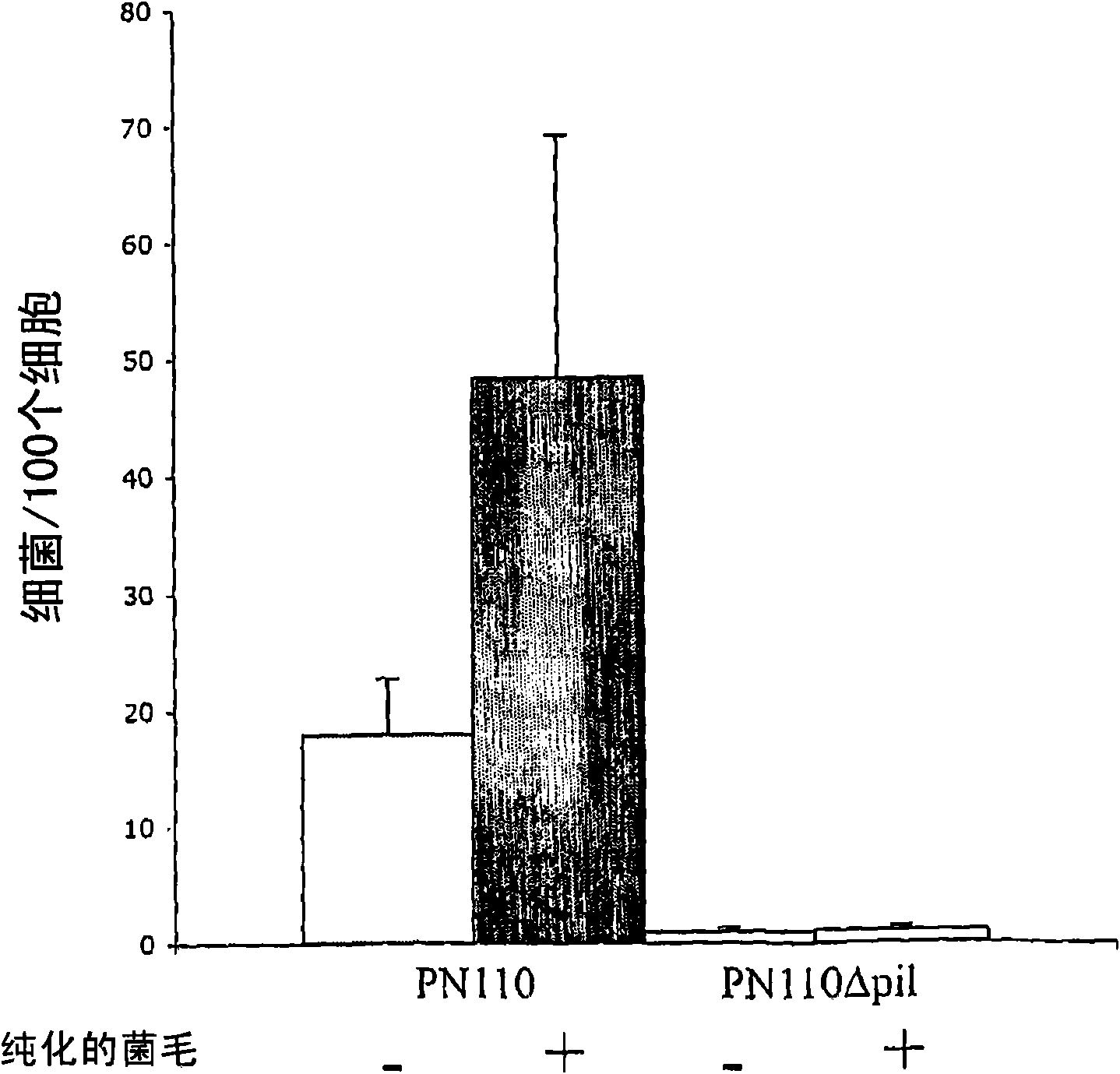
Streptococcus pneumoniae pilus antigens
Polypeptides from Streptococcus pneumoniae are described. In some aspects the polypeptides include pili polypeptides from a second pili island (pilus II island (INVl 04B)) identified in Streptococcus pneumoniae isolate INVl 04. In other aspects the polypeptides include pili polypeptides and non-pilus polypeptides from Streptococcus pneumoniae strains 23F, INV200, and OXC141 that are absent from Streptococcus pneumoniae isolate INV104. The polypeptides, including fragments and variants thereof, may be used in immunogenic compositions for prophylactic or therapeutic immunization against Streptococcus pneumoniae. The polypeptides are also disclosed to be used in compositions useful for the production of antibodies and immunostimulants. Also presented are methods of inhibiting Streptococcus pneumoniae, methods of treating Streptococcus pneumoniae infection, methods of identifying inhibitors of Streptococcus pneumoniae and methods for diagnosing / detecting Streptococcus pneumoniae infection.
Owner:NOVARTIS AG
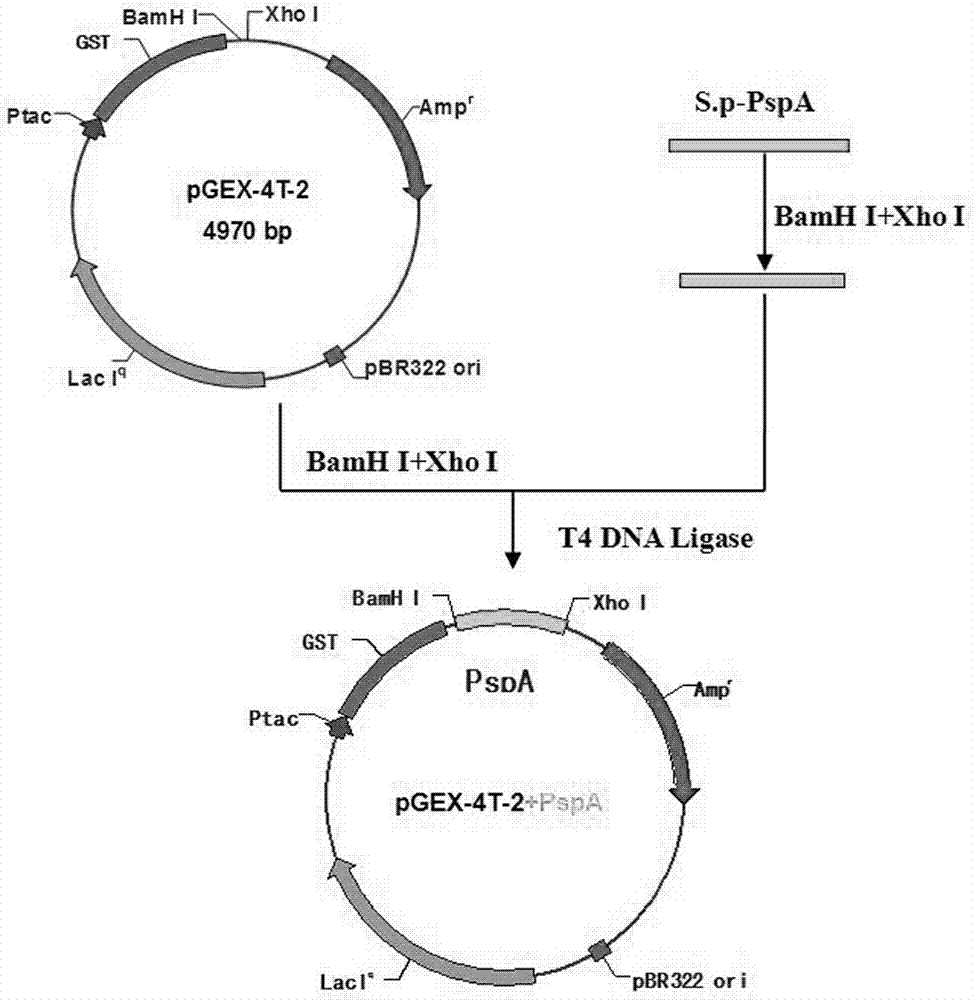
Chemosynthetic extracellular region gene fragment of streptococcus pneumonia PspA protein, and expression and application thereof
ActiveCN103834667AEasy to purifyAvoid interferenceImmunoglobulins against bacteriaMicroorganism based processesGenetic engineeringTGE VACCINE
The invention relates to the fields of genetic engineering technology and diagnostic reagents and provides a chemosynthetic extracellular region gene fragment of streptococcus pneumonia PspA protein and expression and application thereof. According to the invention, strong epitope in streptococcus pneumonia PspA protein is screened through computer analysis, the fragment consisting of the 33rd amino acid to the 109th amino acid, altogether 77 amino acids, is used, codon preferred by prokaryotes is selected, and a brand new gene sequence of the epitope is chemically synthesized; and through usage of genetic engineering technology, the gene fragment is expressed, and the fragment of the strong epitope in streptococcus pneumonia PspA protein is prepared. The expressed protein can be used for research and development of vaccines, detection of streptococcus pneumonia infection antibodies and preparation of monoclonal antibodies and polyclonal antibodies.
Owner:李越希
Application of Toll-like receptor ligand protein in resisting bacterial infection
PendingCN110714000AEnhanced phagocytosisIncrease lethalityAntibacterial agentsBacterial antigen ingredientsCytokineImmunity response
The invention provides an application of a Toll-like receptor ligand protein in resisting bacterial infection. According to the application, streptococcus pneumoniae endopeptidase O (PepO) is a ligandof a Toll-like receptor 2 and a Toll-like receptor 4, can remarkably enhance phagocytosis and killing effects of macrophages on pathogenic bacteria, and can up-regulate secretion of related cytokinesand chemokines, thereby inducing strong innate immune response and enhancing resistance of respiratory tracts to pathogenic bacteria infection.
Owner:CHONGQING MEDICAL UNIVERSITY
Method for purifying streptococcus pneumoniae capsular polysaccharide
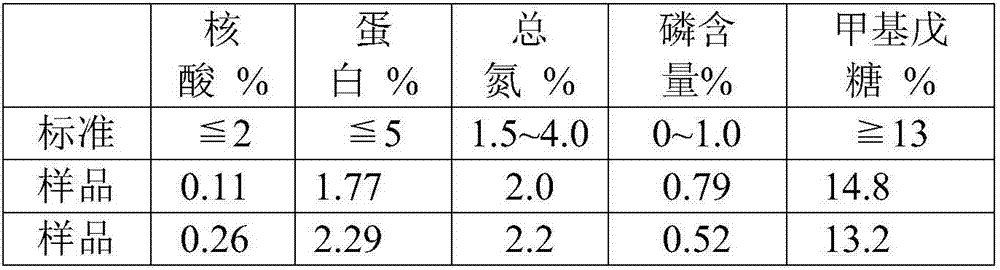
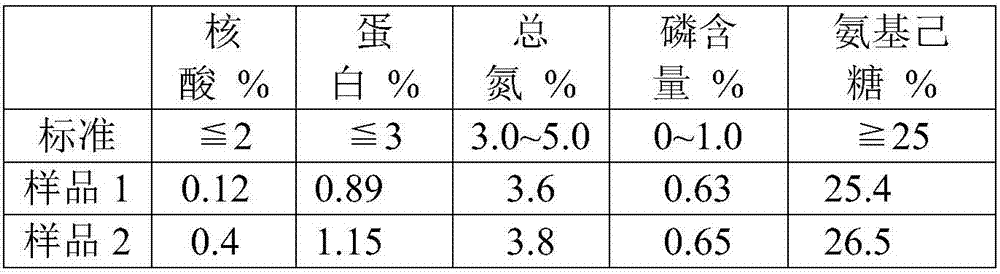
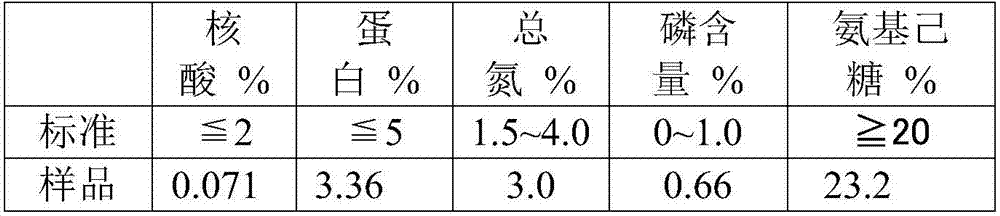
The invention discloses a method for purifying streptococcus pneumoniae capsular polysaccharide. The method comprises 1) inactivating streptococcus pneumoniae, centrifuging the streptococcus pneumoniae and collecting the supernatant, 2) carrying out ultrafiltration and collecting the filtrate, 3) adjusting the pH of the filtrate obtained in step 2) to 3-5, centrifuging the filtrate and collecting the supernatant, (4) adding a calcium salt obtained by the step 3) into the supernatant, adjusting the pH to more than 7, carrying out centrifugation and collecting the supernatant, and 5) adding a precipitant into the supernatant obtained in the step 4), carrying out precipitation, collecting the precipitates, and washing and drying the precipitates so that the purification of the streptococcus pneumoniae capsular polysaccharide is realized. Through the optimization of the purification process, the capsular polysaccharides having protein content, nucleic acid content, sugar content, phosphorus content and nitrogen content satisfying indexes. The method has high content and high yield of streptococcus pneumoniae, simple processes and enlargement easiness and is suitable for the production of a streptococcus pneumoniae polysaccharide vaccine.
Owner:HUALAN BIOLOGICAL ENG INC +2
Kit for simultaneously detecting streptococcus pneumoniae, legionella pneumophila and Moraxella catarrhalis
PendingCN112481401AStrong specificityFast detection methodMicrobiological testing/measurementMicroorganism based processesDisease monitoringMoraxella catarrhalis
Owner:AUTOBIO DIAGNOSTICS CO LTD
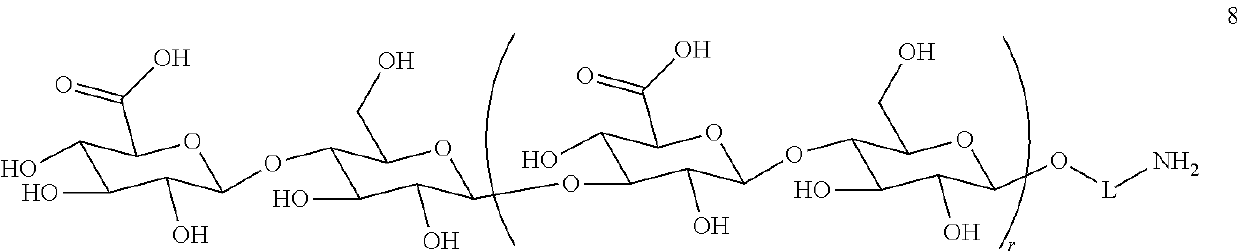
Synthetic vaccines against Streptococcus pneumoniae type 1
ActiveUS10328141B2Improving immunogenicityAdd supportAntibacterial agentsOrganic active ingredientsStreptococcus pneumoniaeDisease
The present invention relates to the total synthesis of saccharide structures contained in the capsular polysaccharide of Streptococcus pneumoniae type 1, to glycoconjugates containing said saccharide structures obtained by total synthesis and to use of such glycoconjugates and pharmaceutical compositions thereof in the immunization against diseases associated with bacteria containing said saccharide structures in their capsular polysaccharide, and more specifically associated with Streptococcus pneumoniae.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Azithromycin eye drops
InactiveCN102579335AAvoid abuseImprove complianceOrganic active ingredientsSenses disorderBacterial ConjunctivitisStaphylococcus aureus
The invention discloses azithromycin eye drops. The azithromycin eye drops are a stable eye preparation which is prepared from azithromycin serving as a main active ingredient and proper auxiliary materials. The long-acting eye drops with mucosa adhesiveness prepared from the latest auxiliary materials at home and abroad have a medicine controlled-release system, the active ingredient stops in eyes for several hours to enhance the antibacterial activity of target tissues, so that the using frequency of the eye drops can be reduced, and the eye drops are convenient to use. The azithromycin eye drops are suitable for treating bacterial conjunctivitis caused by microbial sensitive strains such as G group corynebacterium, haemophilus influenzae, staphylococcus aureus, streptococcus mitis groups and streptococcus pneumoniae.
Owner:GUANGDONG WHOLEWIN TECH
Improved preparation of vaccines against streptococcus pneumoniae type 3
InactiveUS20190119313A1Easy to installIncrease productionAntibacterial agentsSugar derivativesDiseaseStreptococcus pneumoniae
The present invention relates to the preparation of a synthetic tetrasaccharide, hexasaccharide and octasaccharide representing part of the repeating unit of the Streptococcus pneumoniae type 3 capsular polysaccharide as well as conjugates thereof. Said conjugates are particularly useful for prevention and / or treatment of diseases associated with Streptococcus pneumoniae, and more specifically of diseases associated with Streptococcus pneumoniae type 3. The disclosed synthetic method has the huge advantages over the state of the art synthetic methods that intermediate products are crystalline, coupling reaction yields are higher, less reaction steps are required and purification of intermediate products is easier.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
Application of deoxyshikonin
InactiveCN103622937AAntibacterialObvious concentration dependenceAntibacterial agentsHeterocyclic compound active ingredientsStaphylococcus aureusPenicillin resistant
The invention discloses application of deoxyshikonin (5,8-dihydroxy-2-(4-methyl-3-phenyl)-1,4-naphthalenedione) as a histidine kinases VicK inhibitor in vitro or to preparation of the histidine kinases VicK inhibitor. Deoxyshikonin exerts certain antibacterial action on in-vivo streptococcus pneumonia and clinical penicillin-resistant streptococcus pneumonia; moreover, combined deoxyshikonin and penicillin exert a good antibacterial effect on streptococcus pneumonia and has substantial bacteriostatic action on staphylococcus aureus.
Owner:CHONGQING MEDICAL UNIVERSITY
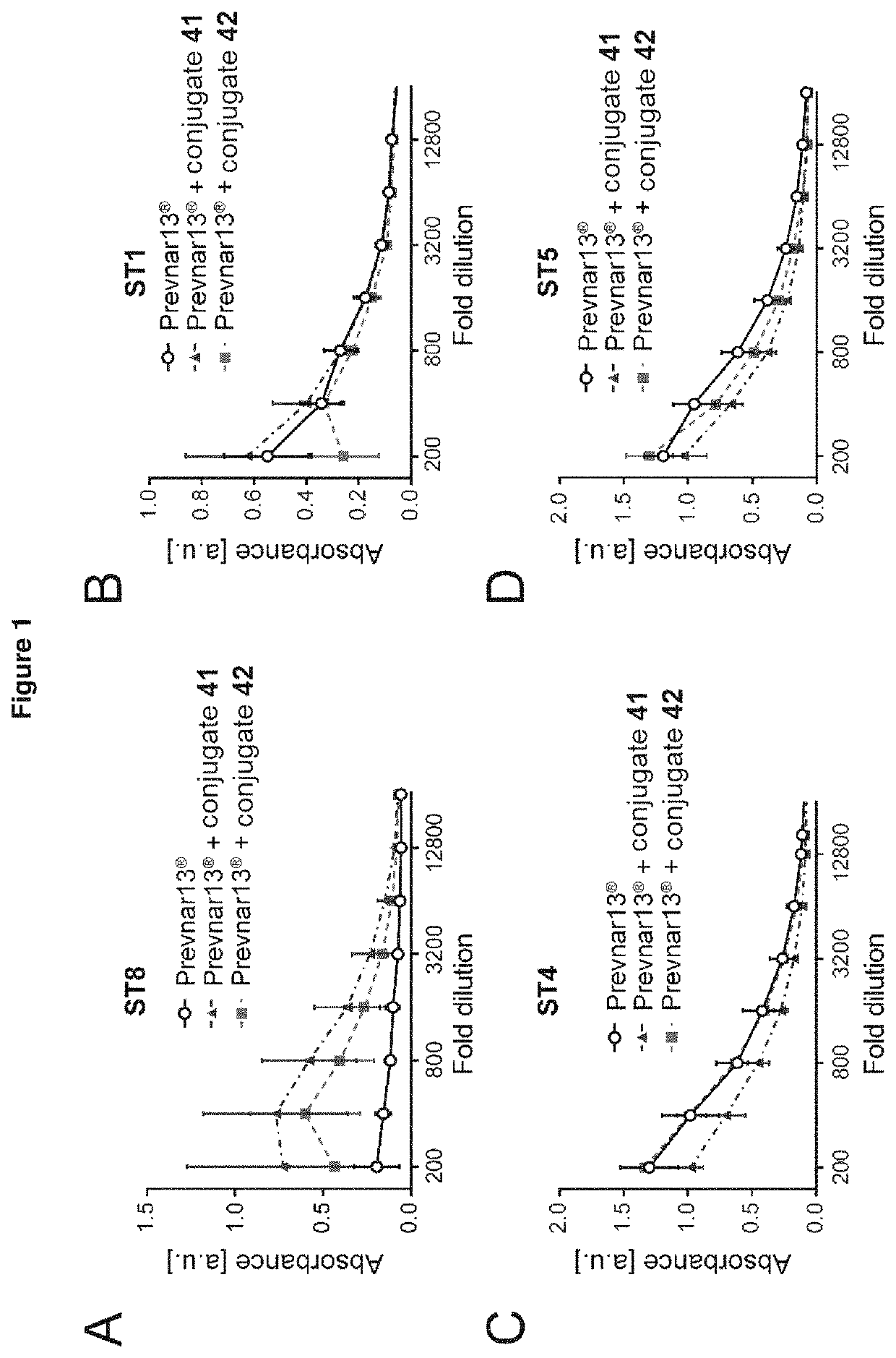
Pneumococcal polysaccharide-protein conjugate composition
ActiveUS11191822B2Strong immune responseImpairing immune responseAntibacterial agentsBacterial antigen ingredientsTetanus toxoidsSerotype
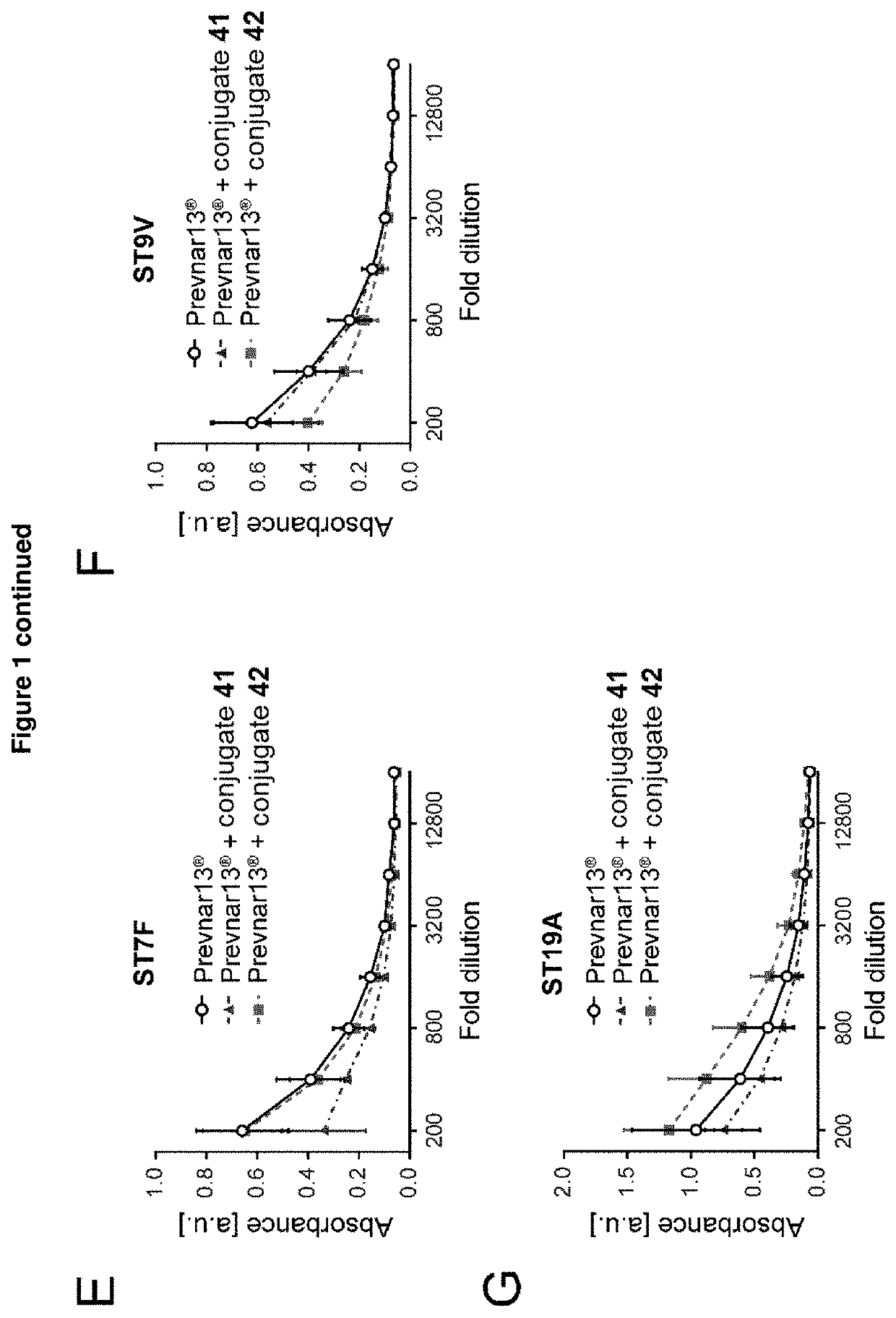
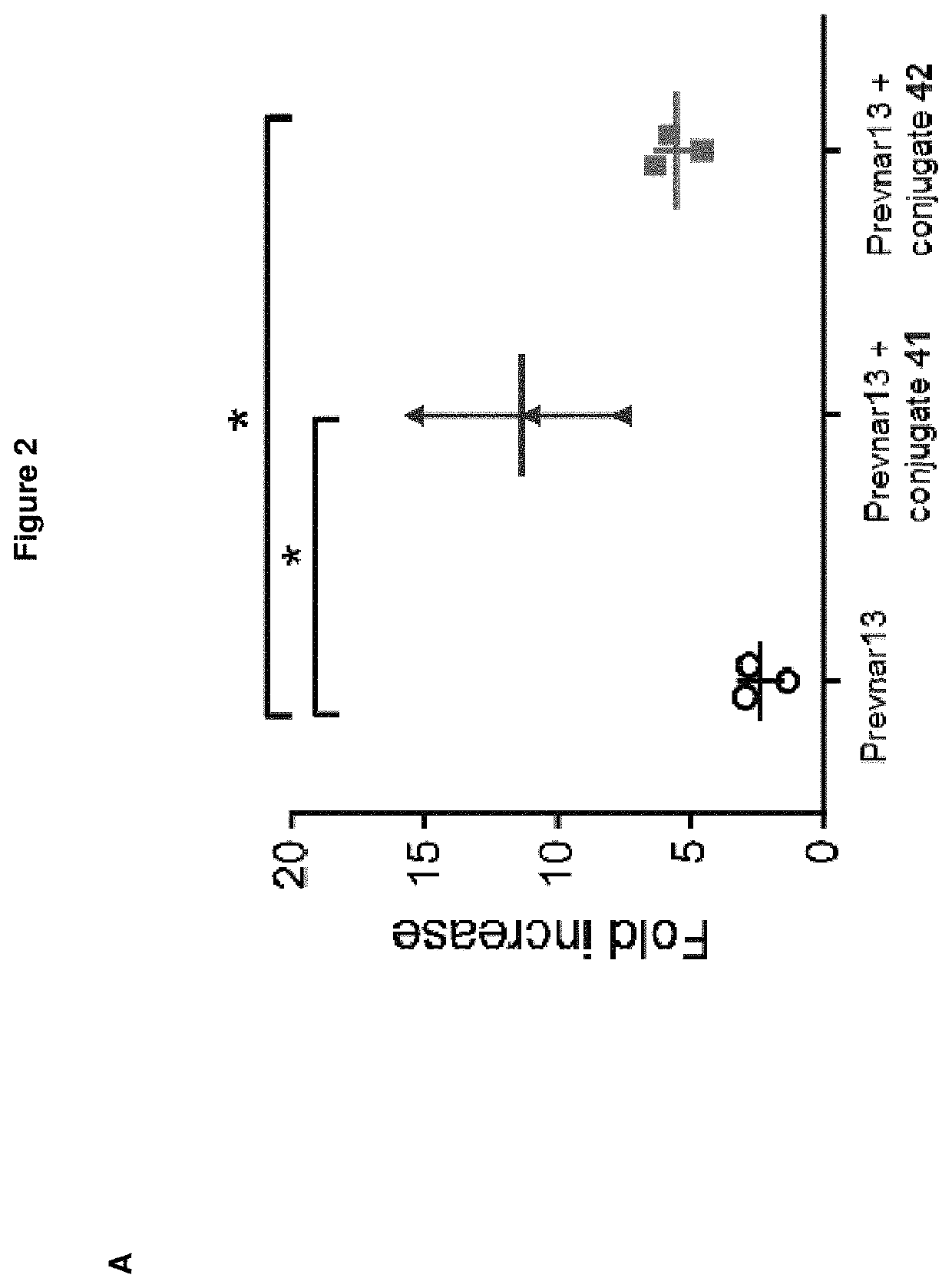
The present invention relates to immunogenic compositions comprising a conjugate of a saccharide from Streptococcus pneumoniae serotype 8 and a carrier protein, and a mixture consisting of capsular polysaccharides from Streptococcus pneumoniae serotypes 1, 3, 4, 5, 6A, 6B, 7F, 9V, 14, 18C, 19A, 19F and 23F individually conjugated to CRM197 carrier protein, or a mixture consisting of capsular polysaccharides from Streptococcus pneumoniae serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F and 23F individually conjugated to a carrier protein, wherein the capsular polysaccharides from Streptococcus pneumoniae serotypes 1, 4, 5, 6B, 7F, 9V, 14, and 23F are individually conjugated to protein D, the capsular polysaccharide from Streptococcus pneumoniae serotype 18C is conjugated to tetanus toxoid and the capsular polysaccharide from Streptococcus pneumoniae serotype 19F is conjugated to diphtheria toxoid. Said compositions are useful for the prevention and / or treatment of diseases caused by Streptococcus pneumoniae.
Owner:MAX PLANCK GESELLSCHAFT ZUR FOERDERUNG DER WISSENSCHAFTEN EV
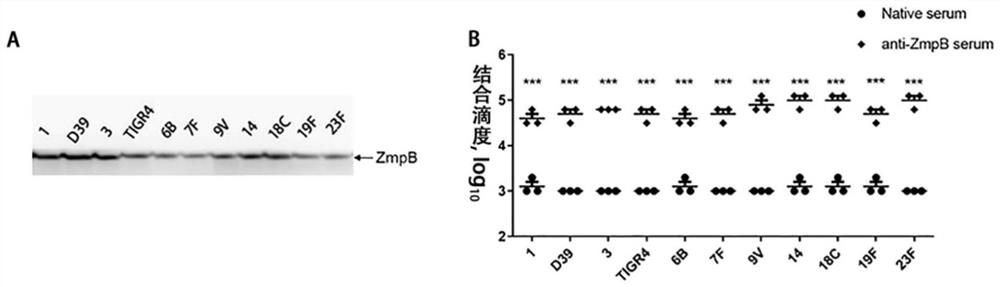
Application of Streptococcus pneumoniae protein in resisting Streptococcus pneumoniae infection
ActiveCN109456393BIncrease infectionReduced Colonization Protection ExperimentAntibacterial agentsBacterial antigen ingredientsStreptococcus infectionAmino acid peptide
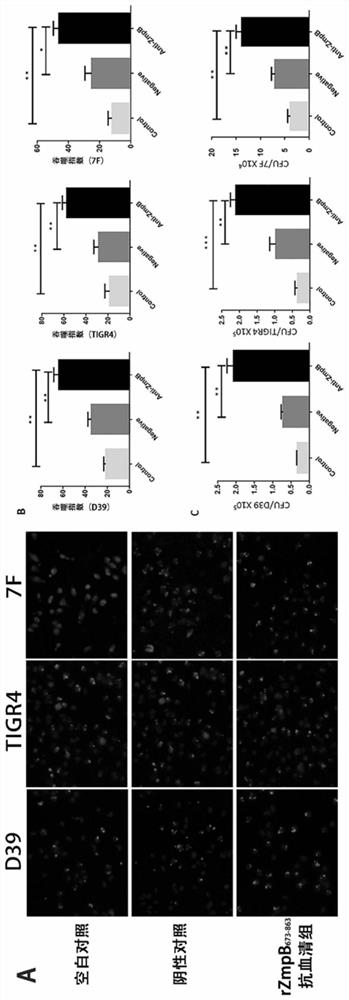
The invention provides the application of Streptococcus pneumoniae protein in resisting Streptococcus pneumoniae infection. The Streptococcus pneumoniae endopeptidase O (PepO) of the present invention is a subcutaneous immune adjuvant, mixed and fused with the 673rd to 863rd amino acid peptide of zinc metalloprotease B (ZmpB), and the prepared Streptococcus pneumoniae Protein vaccines have a good protective effect against Streptococcus pneumoniae infection.
Owner:CHONGQING MEDICAL UNIVERSITY
Identification of metabolomic signatures in urine samples for tuberculosis diagnosis
PendingUS20210278405A1Magnetic measurementsAnalysis using nuclear magnetic resonanceNon invasiveUrine sample
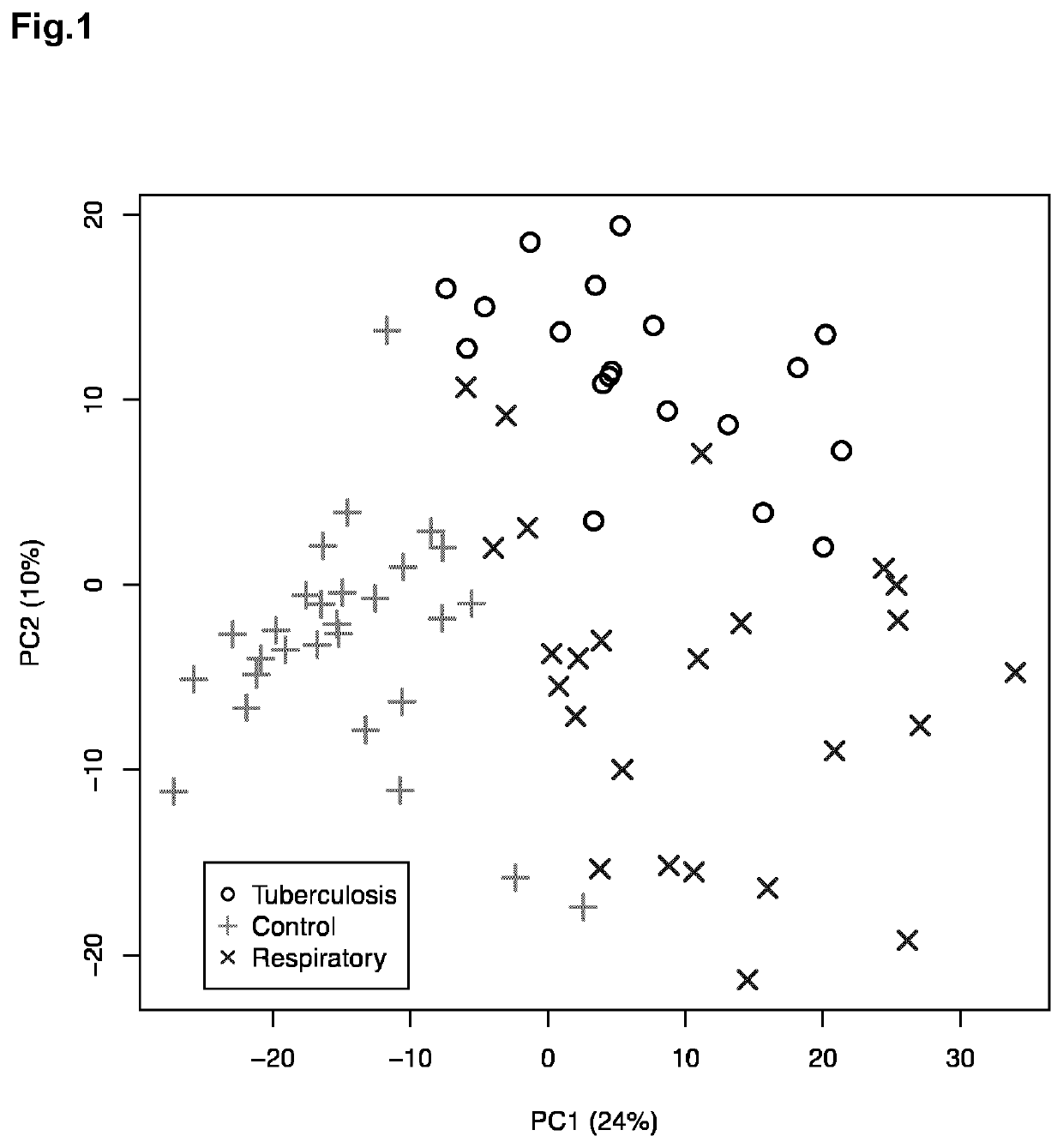
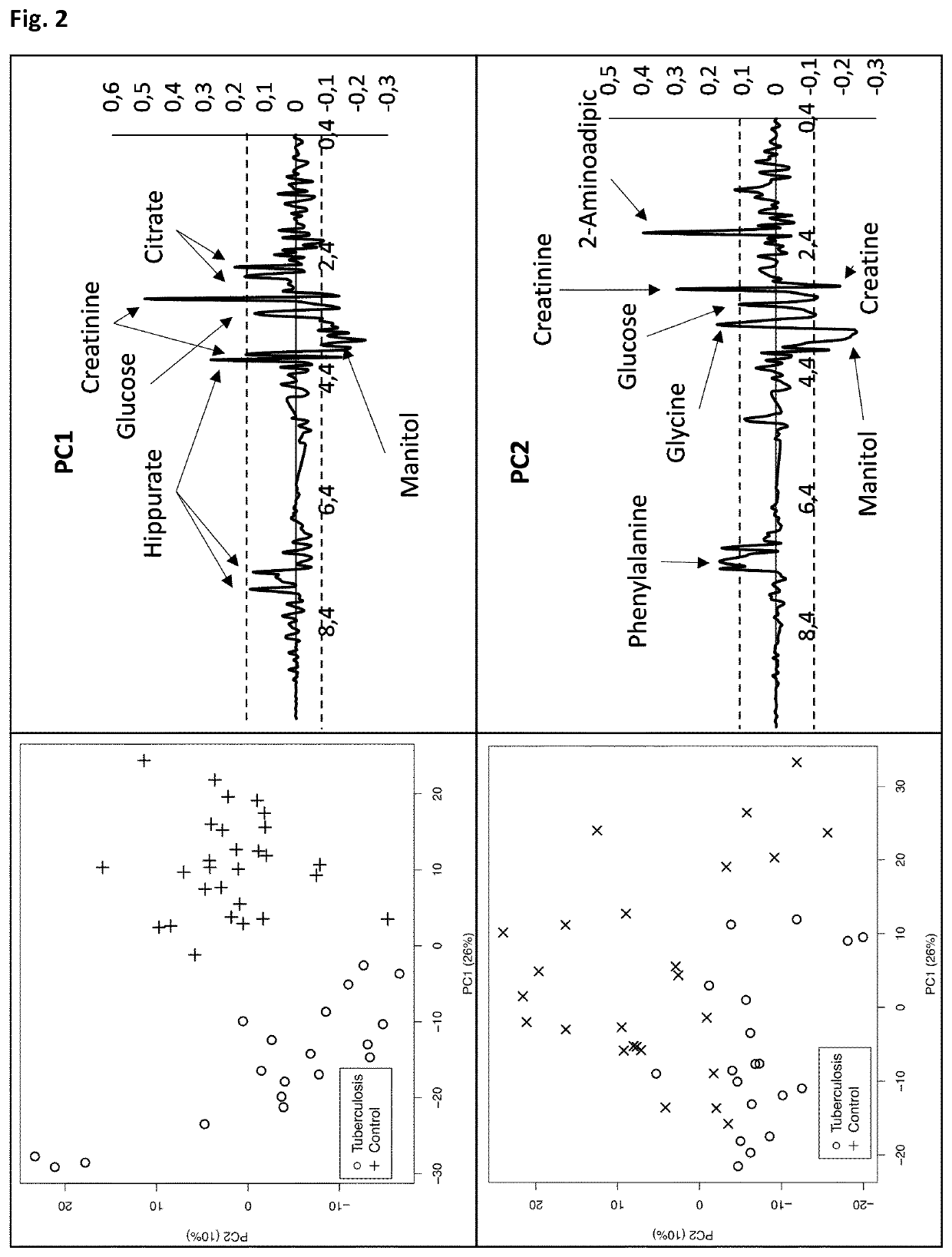
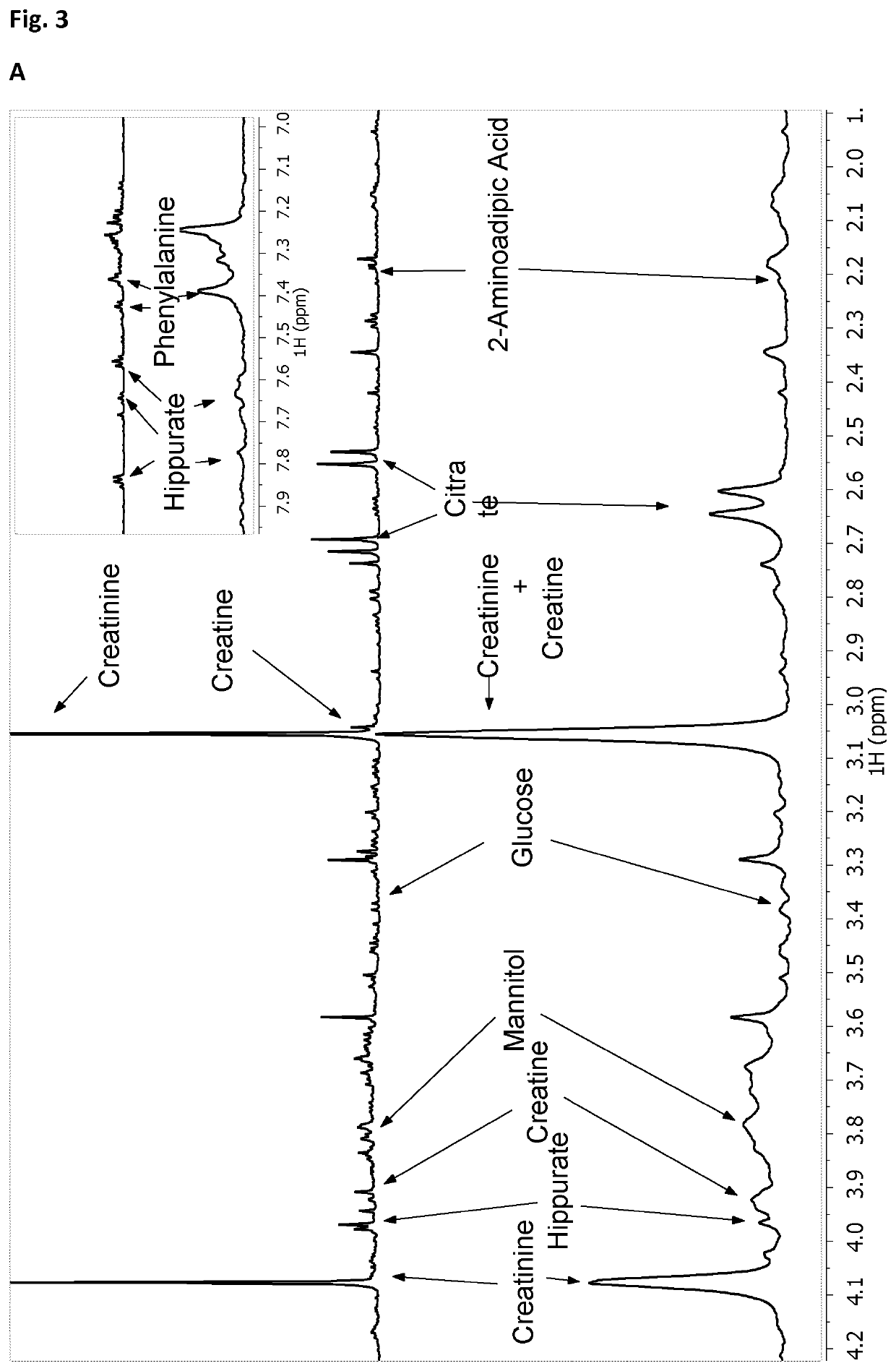
The authors of the present invention have identified a series of metabolic markers present in the urine samples collected from patients diagnosed of tuberculosis (TB, n=19), respiratory infections caused by Streptococcus pneumoniae (R1, n=25) and healthy controls (HC, n=29). These metabolic markers selected are significantly differentiated between Healthy Controls (HC) and patients diagnosed of tuberculosis, between tuberculosis patients versus patients affected by respiratory infections caused by S. pneumoniae, and between patients affected by respiratory infections caused by S. pneumoniae and HC. These metabolic markers can thus be used in a non-invasive diagnostic method identifying and classifying patients.
Owner:FUNDACIO INST DINVESTIGACIO & CIENCIES DE LA SALUT GERMANS TRIAS I PUJOL
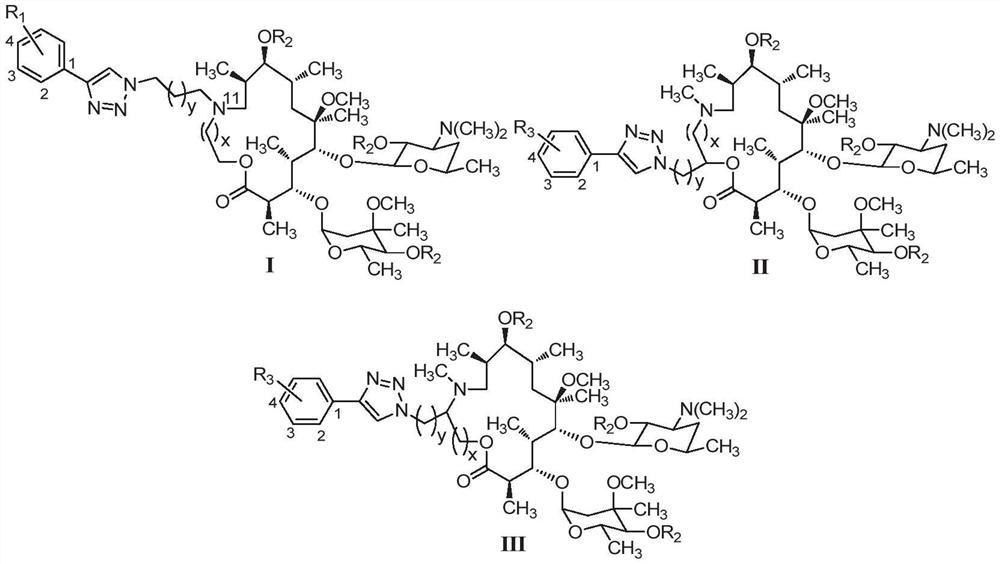
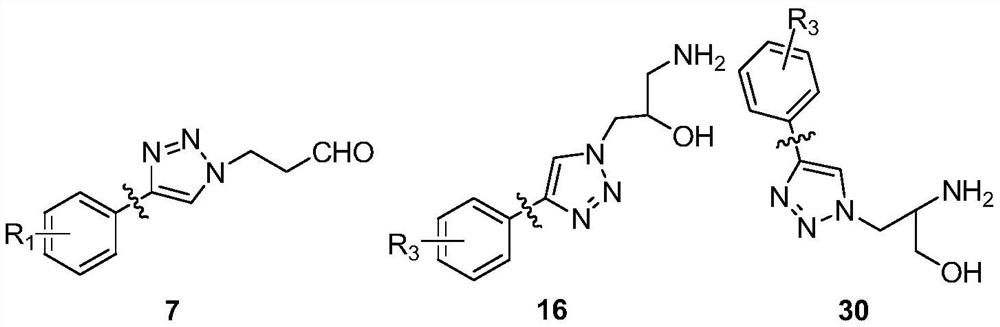
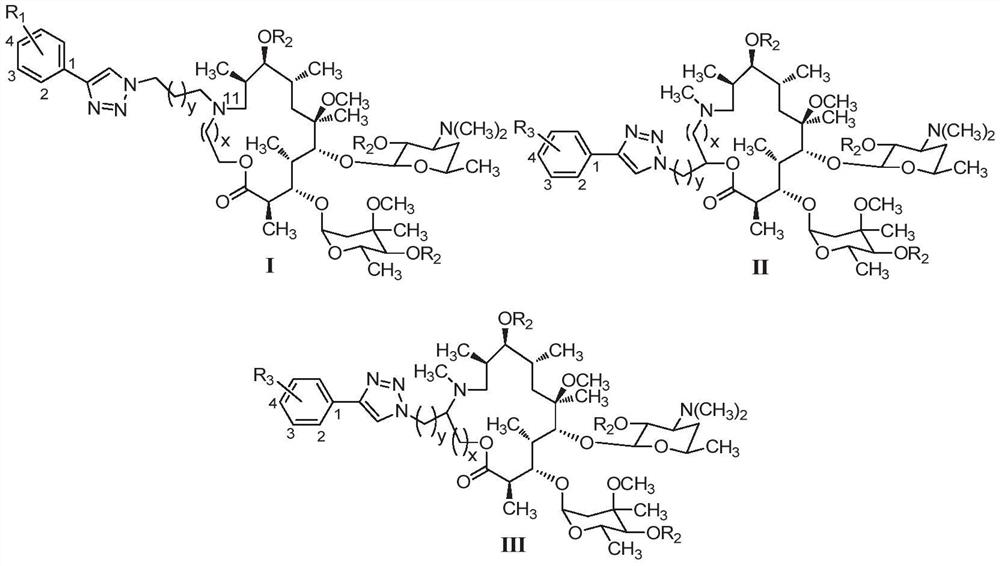
15-membered clarithromycin derivative as well as preparation method and application thereof
PendingCN112876525AHigh antibacterial activityAntibacterial agentsOrganic active ingredientsStreptococcus pyogenesPhenacyl
The invention discloses a 15-membered clarithromycin derivative as well as a preparation method and application thereof. The 15-membered clarithromycin derivative has a structure as shown in a general formula I, II or III (shown in the description), wherein R1 is selected from hydrogen, saturated or unsaturated alkyl, saturated or unsaturated alkoxy and halogen; R2 is selected from hydrogen, an acetyl group, a benzoyl group or a triethyl siloxy group; R3 is selected from a saturated or unsaturated alkyl group and a saturated or unsaturated alkoxy group; x is 1; and y is 1. The 15-membered clarithromycin derivative provided by the invention has excellent antibacterial activity, particularly has a good inhibition effect on drug-resistant staphylococcus aureus, ermB drug-resistant streptococcus pneumoniae, ermB + mefA drug-resistant streptococcus pneumoniae and drug-resistant streptococcus pyogenes, and can be used for preparing drugs for treating bacterial infection.
Owner:SHANDONG UNIV
Streptococcus pneumoniae antiserum without cross-reactivity and method for producing the same
PendingUS20220127339A1Strong specificityAntibacterial agentsSerum immunoglobulinsCMV PneumoniaSerotype
The present invention relates to a Streptococcus pneumoniae antiserum without cross-reactivity and method for producing the same, more specifically, it relates to a method for producing a S. pneumoniae antiserum comprising the step of removing cross-reactivity using S. pneumoniae and a S. pneumoniae antiserum prepared by the method. The Streptococcus pneumoniae antiserum prepared according to the method of the present invention has very high specificity for a particular serotype, since the cross-reactivity with S. pneumoniae of serotypes expressing capsular polysaccharides of similar structure is removed. Therefore, it can be very useful in the related art that requires accurate quantification of S. pneumoniae capsular polysaccharide.
Owner:SK BIOSCI CO LTD
Streptococcus pneumoniae autolysin small fragment gene expression protein, preparation method and application thereof
InactiveCN104152467AEasy to operateLow costAntibacterial agentsPeptide/protein ingredientsCloning vectorAmino acid
The invention discloses a streptococcus pneumoniae autolysin small fragment gene expression protein as well as a preparation method and application thereof. The small fragment gene expression protein has the amino acid sequence shown in SEQIDNO:5. The preparation method comprises the steps of extracting the genome DNA of streptococcus pneumoniae (ATCC49619); by taking the obtained DNA as a template, carrying out PCR with a PCR primer having two restriction enzyme cutting sites BamHI and HindIII; splicing the PCR product recycled in glue cutting with a cloning vector to build a clone plasmid; and cutting glue to recycle 500bp small fragments through the clone plasmid built through double digestion respectively by BamHI and HindIII, connecting by a ligase and an expression vector, then converting into a competent cell, picking one and cloning, building a recombination expression plasmid, carrying out IPTG inducible expression, and recycling and purifying the protein. The streptococcus pneumoniae autolysin small fragment gene expression protein is used in preparation of antibacterial medicines.
Owner:DALIAN MEDICAL UNIVERSITY
Dedicated diluent for detecting streptococcus pneumoniae in urine sample
ActiveCN109596823AStable soluble stateFast and effective dissociationMaterial analysisStreptococcus pneumoniaeAdditive ingredient
The invention discloses a dedicated diluent for detecting streptococcus pneumoniae in a urine sample. The diluent consists of five ingredients, including borax, 1307Prill, [Epsilon]-polylysine, a C-reactive protein polyclonal antibody and purified water, wherein the content of each ingredient is as follows: the content of the borax which serves as a first ingredient is 50-100mM, the content of 1307Prill which serves as a second ingredient is 0.5-1%, the content of the [Epsilon]-polylysine which serves as a third ingredient is 0.5-1%, the content of the C-reactive protein polyclonal antibody which serves as a fourth ingredient is 10-20ug / ml, and the balance is purified water. After the diluent is mixed with the urine sample at a volume ratio of 1:9, the interference of C-reactive protein inthe urine sample for a streptococcus pneumoniae detection result can be effectively inhibited, the detection rate of the streptococcus pneumoniae is obviously improved so as to perform significance for accurately detecting the streptococcus pneumoniae in the urine sample, and therefore, the diluent has a good clinical application value.
Owner:HANGZHOU BEACONLAB BIOTECH CO LTD
Chemical Synthesis, Expression and Application of the Gene Fragment of Streptococcus Pneumoniae Surface Adhesin-a
ActiveCN103773779BEasy to purifyAvoid interferenceImmunoglobulins against bacteriaMicroorganism based processesChemical synthesisGenetic engineering
Owner:中国人民解放军东部战区疾病预防控制中心
Anti-human Streptococcus pneumoniae fam2 family pspa protein antibody and immunochromatographic kit using the antibody
ActiveCN105585634BStrong specificityHigh purityBiological material analysisImmunoglobulins against bacteriaStreptococcus pneumoniaeEpitope
The invention relates to antibodies for resisting human streptococcus pneumoniae fam2 family PspA protein and an immunochromatographic kit applying the antibodies to detection on human streptococcus pneumoniae. The antibodies for resisting the human streptococcus pneumoniae fam2 family PspA protein are used for recognizing two linear antigenic epitopes composed of 34-47 amino acids and 274-287 amino acids of the human streptococcus pneumoniae fam2 family PspA protein respectively, and the sequence number of the human streptococcus pneumoniae fam2 family PspA protein in GenBank is WP_054380072.1; the sequences of the 34-47 amino acids and the 274-287 amino acids of the human streptococcus pneumoniae fam2 family PspA protein are SPQVVEKSSLEKKY and KLLDSLDPEGKTQD respectively. The two rabbit antibodies for resisting the human streptococcus pneumoniae fam2 family PspA protein have the advantages of being good in specificity, high in purity and titer and low in preparation cost.
Owner:HUBEI UNIV OF TECH +1
A kind of hybrid compound of macrolide and quinolone and preparation method thereof
ActiveCN109942654BHigh activityAdapt to working productionAntibacterial agentsOrganic active ingredientsStreptococcus pyogenesMacrocyclic lactone
The present invention provides a macrocyclic lactone and quinolone hybrid, characterized in that the macrocyclic lactone and quinolone hybrid include compounds with general formula I and general formula II, or, the macrocyclic lactone and quinolone hybrids include pharmaceutically acceptable salts formed by compounds of the general formula I and II and inorganic acids or organic acids, and the macrolide and quinolone hybrids can be better adapted to industrial production , and has good anti-susceptible and anti-drug resistance against common clinical erythromycin-resistant Streptococcus pneumoniae, Staphylococcus aureus, Streptococcus pyogenes, Mora catarrhalis and Haemophilus influenzae Bacterial activity, can effectively treat clinical bacterial pneumonia or pneumonia caused by other microorganisms (such as mycoplasma, Legionella, etc.), and other tissue infections;
Owner:BEIJING INSTITUTE OF TECHNOLOGYGY
Application of sanguinarine in inhibiting growth of streptococcus pneumonia
PendingCN111166749AGrowth inhibitionDefinite inhibitory effectAntibacterial agentsOrganic active ingredientsMicrobiologySanguinarine
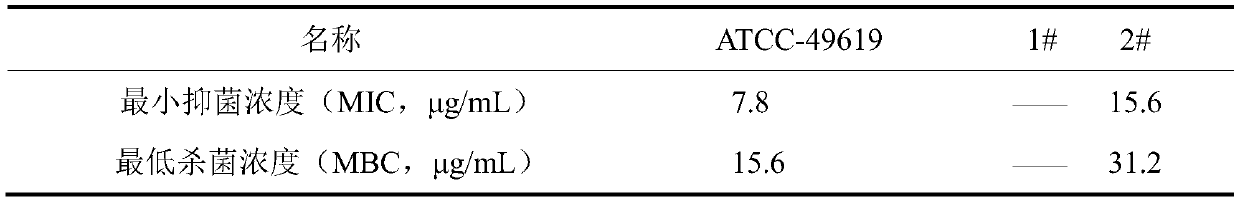
The invention discloses an application of sanguinarine in inhibiting the growth of streptococcus pneumonia, according to the fact that the sanguinarine has a good in-vitro killing effect on streptococcus pneumonia, the sanguinarine can inhibit the growth of streptococcus pneumonia, the minimum bactericidal concentration is 31.2 mu g / mL, and the minimum inhibitory concentration is 15.6 mu g / mL. Thesanguinarine provided by the invention has an inhibition effect on pneumonia streptococcus, and has a wide application value in the fields of medicines and the like.
Owner:SHAANXI UNIV OF SCI & TECH
Use of short-chain fatty acids for the treatment of bacterial superinfections post-influenza
PendingUS20210038545A1Increase infectionIncreased susceptibilityAntibacterial agentsVaccination/ovulation diagnosticsIntestinal microorganismsSevere influenza
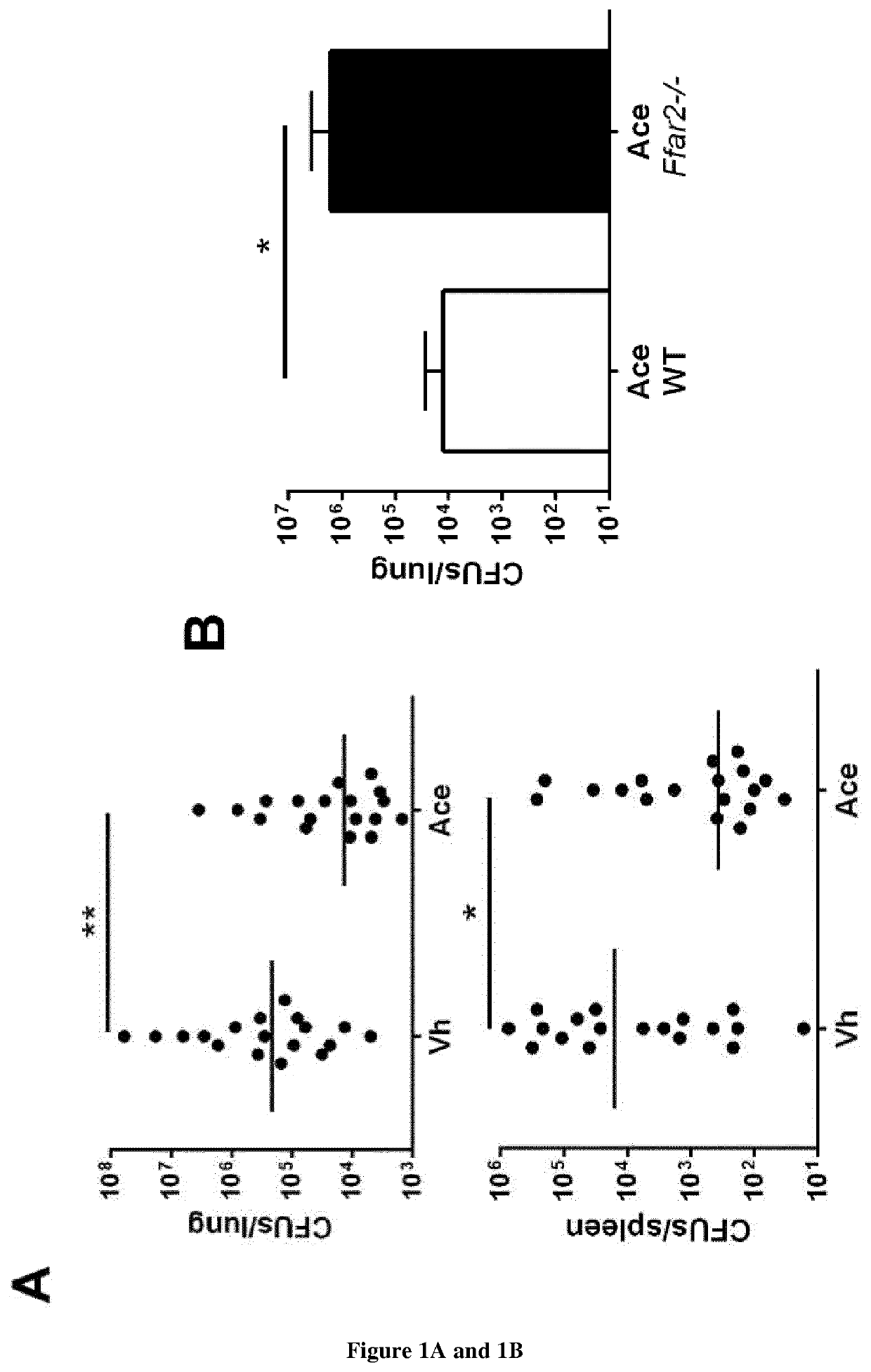
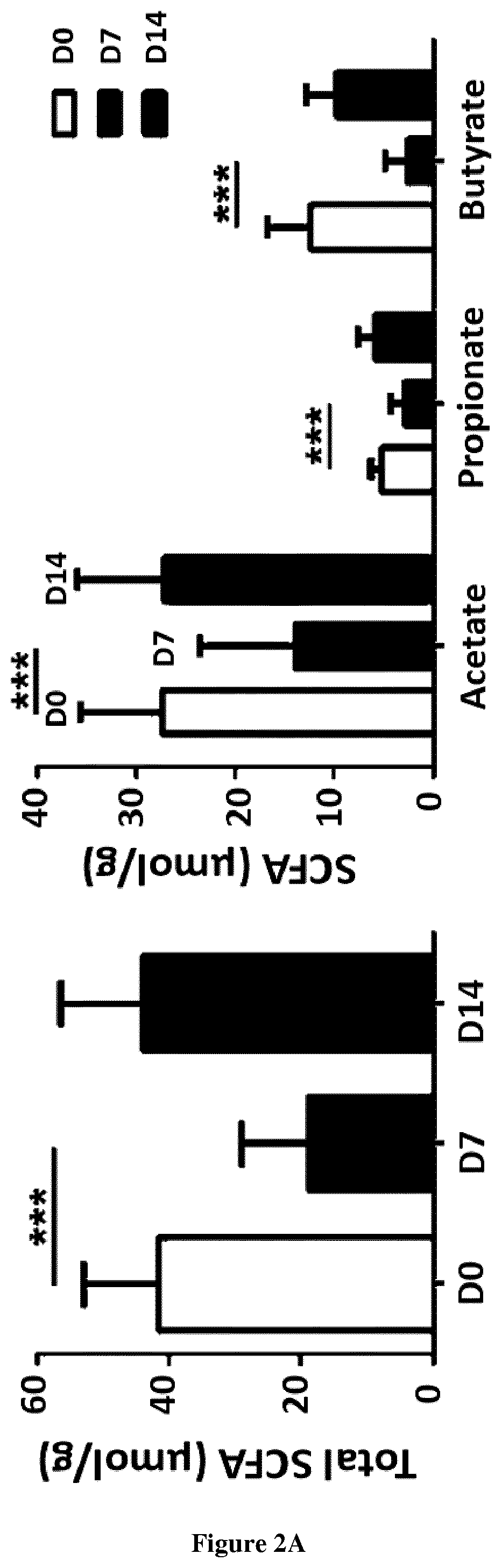
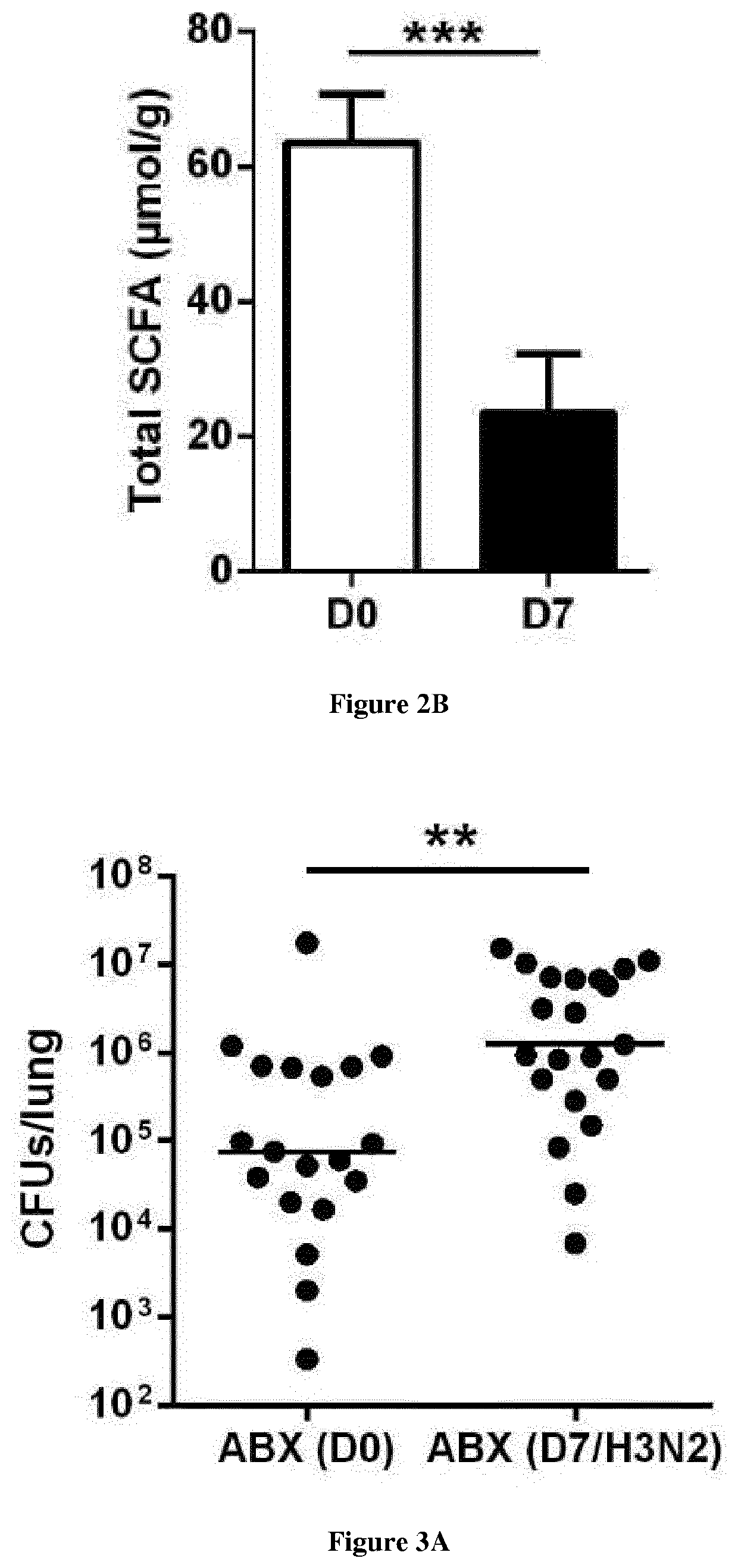
Severe influenza is associated with defects in pulmonary innate immunity, a phenomenon leading to secondary bacterial infections. The gut microbiota can control immune / inflammatory responses locally and at distant sites. The inventors hypothesized that perturbation of the gut microbiota during severe influenza might participate in bacterial superinfection post-influenza. Their data demonstrated that influenza infection profoundly altered the functionality of the gut microbiota as assessed by the altered production of short chain fatty acids (SCFAs). Remarkably, treatment of colonized (IAV microbiota) mice or IAV-infected mice with acetate, the main SCFA found systematically, reinforced host defenses against S. pneumoniae. The present invention thus relates to the use of short-chain fatty acids for the treatment of bacterial superinfections post-influenza.
Owner:INST NAT DE LA SANTE & DE LA RECHERCHE MEDICALE (INSERM) +4
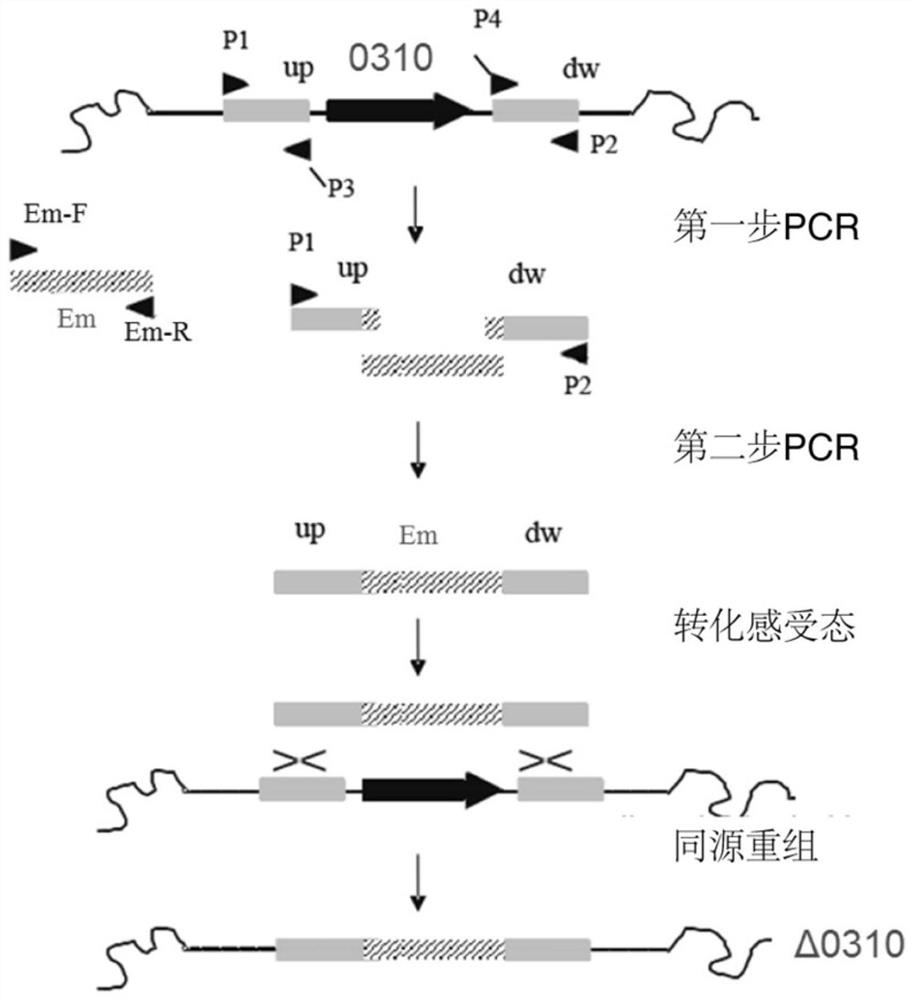
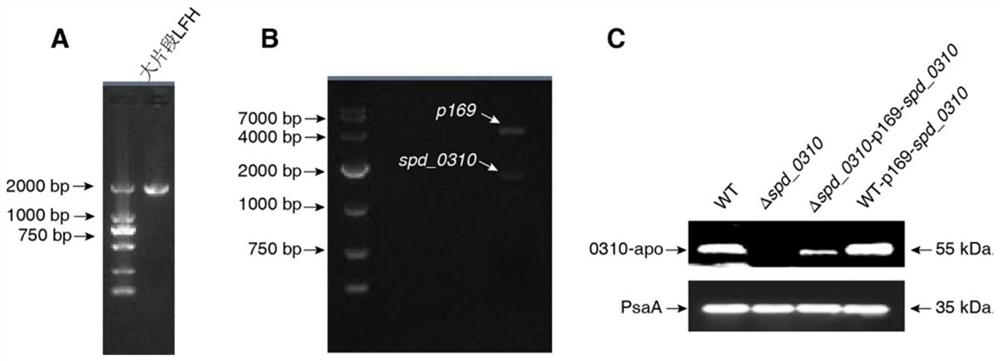
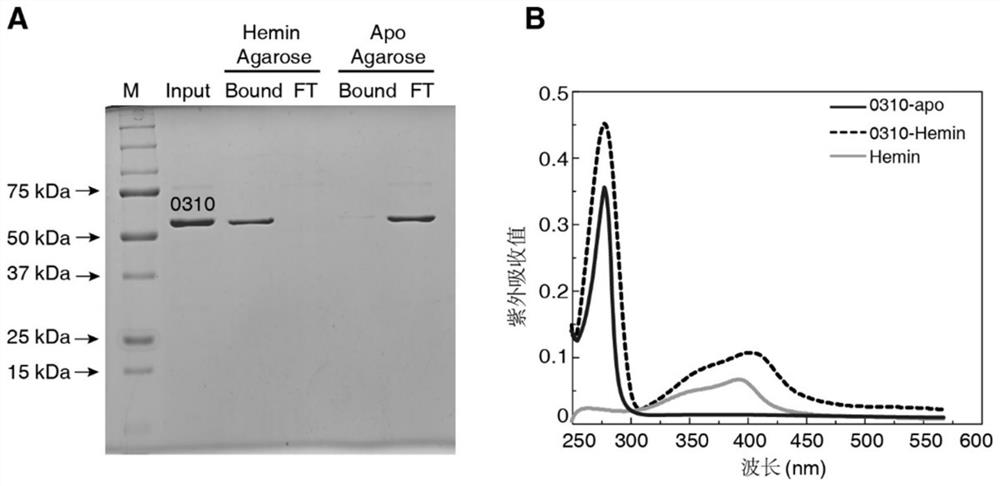
Application of spd_0310 protein as a target in the preparation of drugs for preventing and treating Streptococcus pneumoniae infection
ActiveCN110812484BReduce conservatismHighly conservativeAntibacterial agentsOrganic active ingredientsAntimicrobial drugStreptococcus infection
The invention discloses the application of SPD_0310 protein as a target in the preparation of medicines for preventing and treating Streptococcus pneumoniae infection. In the present invention, it was found that the expression level of SPD_0310 in the triple mutant strain in which the three main iron transport genes of Streptococcus pneumoniae were knocked out at the same time was increased and could grow normally, indicating that the SPD_0310 protein may be involved in the interaction of various iron transport proteins. The present invention also found that the spd_0310 gene knockout strain has reduced infectivity to mice. Based on this, SPD_0310 can be used to prepare antibodies to the protein, so as to facilitate the development of antibody drugs. In addition, the present invention also found that the active center of the protein can bind heme, and proved that porphyrins can be used as substrates to compete with heme, indicating that porphyrins can be used as inhibitors of SPD_0310 to inhibit Streptococcus pneumoniae toxicity, which has broad application prospects in the field of antibacterial drugs.
Owner:JINAN UNIVERSITY
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com