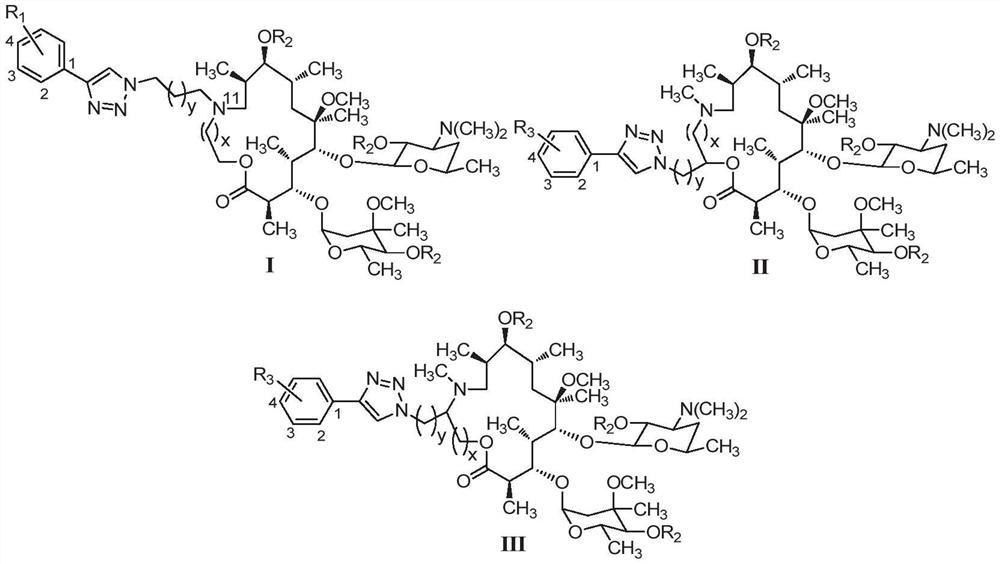
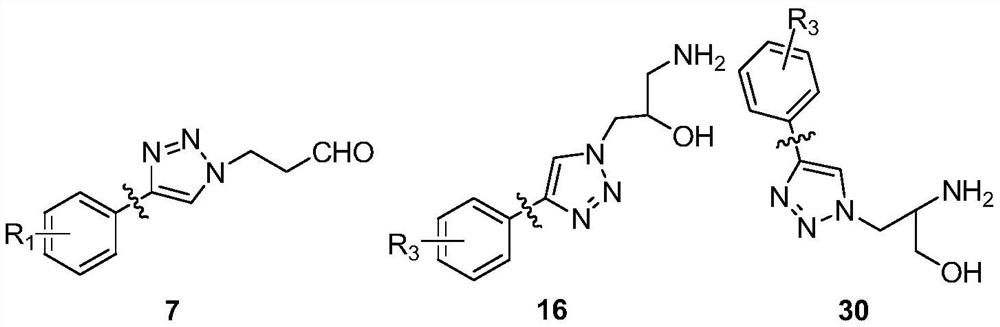
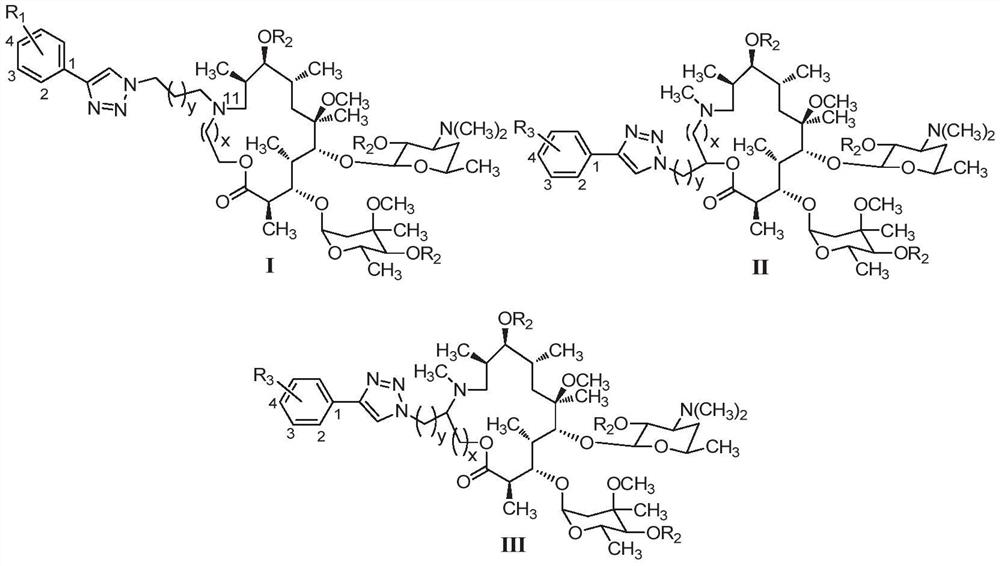
15-membered clarithromycin derivative as well as preparation method and application thereof
A technology of clarithromycin and hydroxyclarithromycin, which is applied in the preparation of sugar derivatives, sugar derivatives, sugar derivatives, etc., can solve the problems of lack of chemical reactivity and difficult structural modification.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0172] Preparation of 9(S)-hydroxy-6-O-methylerythromycin A(2)
[0173] Dissolve clarithromycin (20 g, 26.8 mmol) in a mixed solvent of anhydrous tetrahydrofuran (150 mL) and methanol (300 mL). In an ice bath, dissolve NaBH within 1 h 4 (6 g, 158.6 mmol) was added to the above solution in 3 batches. After the addition was complete, the reaction solution was stirred at room temperature for 24 h. After the reaction was complete, the reaction solution was concentrated, and then DCM (200 mL) and saturated NH 4 Cl solution (200 mL) was added to the concentrated residue. Stir well, stand still for liquid separation, wash the aqueous phase with DCM (100 mL×2), and combine the organic phases. The organic phase was anhydrous Na 2 SO 4 Dry, filter with suction, concentrate to obtain the crude product, and then through column chromatography (DCM / CH 3 OH / NH 3 ·H 2 O=30:1:0.1~20:1:0.1~10:1:0.1) isolated to obtain 10.5 g of white solid with a yield of 52%. Melting point 208–210°C;...
Embodiment 2
[0175] Preparation of 2',4",9(S)-triethylsilyloxy-6-O-methylerythromycin A(3)
[0176] 9(S)-Hydroxy-6-O-methylerythromycin A (2) (8 g, 10.7 mmol) and imidazole (7.2 g, 105.8 mmol) were dissolved in N,N-dimethylformamide (100 mL) middle. Under an ice bath, triethylchlorosilane (5.5 g, 36.5 mmol) was slowly added dropwise to the above solution. After the drop was completed, the reaction was stirred at room temperature for 18 h under the protection of nitrogen. After the reaction is complete, add water (100mL) to the reaction solution to quench the reaction, then add EtOAc (100mL) and n-hexane (100mL), stir evenly, leave to separate the liquids, and the organic phase is washed with saturated NH 4 Cl solution (100 mL) washed. The organic phase was anhydrous Na 2 SO 4 Dry, filter with suction, and concentrate to obtain a crude product, which is then separated by column chromatography (petroleum ether / acetone = 80:1-50:1-30:1-10:1) to obtain 7.9 g of a white foamy solid with a y...
Embodiment 3
[0178] Preparation of ring-opening intermediate (6)
[0179] 2',4",9(S)-triethylsilyloxy-6-O-methylerythromycin A (3) (2g, 1.83mmol) was dissolved in chloroform (20mL). Under ice bath, Lead tetraacetate (0.97g, 2.19mmol) was slowly added to the above solution, and after the addition was complete, the reaction solution was stirred and reacted for 0.5h in an ice bath to obtain a chloroform solution of the ring-opening intermediate (4). Under an ice bath, ethanolamine (5 ) (224mg, 3.67mmol) and sodium triacetoxyborohydride (1.16g, 5.47mmol) were slowly added to the above solution, after the addition was complete, the reaction solution was stirred at room temperature for 4h. After the reaction was completed, saturated NaHCO was added to the reaction solution 3 (20 mL) solution to quench the reaction. Stir evenly, let stand to separate the layers, wash the aqueous phase with DCM (20 mL×2), and combine the organic phases. The organic phase was anhydrous Na 2 SO 4 Dry, filter wit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com