Aspirin composition as well as preparation method and application thereof
A technology of aspirin and composition, which is applied in the field of aspirin composition and its preparation, can solve problems such as difficult to realize drugs, and achieve the effect of solving stomach irritation and difficulty swallowing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
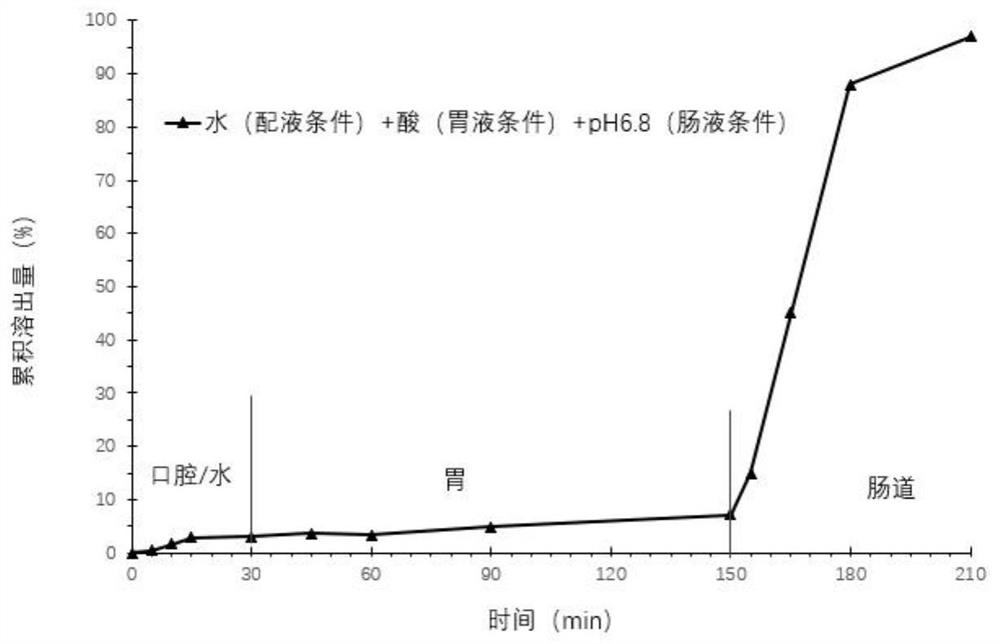
Image
Examples
Embodiment 1
[0050] This embodiment provides a kind of aspirin composition, and its preparation method comprises the following steps:
[0051] S1. Aspirin is used as the raw material drug; wherein, 80% of the particle size of the raw material drug can be controlled between 80-100 mesh.
[0052] S2. Add 50g of hydroxypropyl cellulose (HPC-SL) to the mixed solvent of 50g of water and 400g of ethanol while stirring, and keep stirring until the solution is clear, then add 50g of talcum powder and stir to prepare a suspension solution to obtain the first isolation coating solution and set aside.
[0053] S3, placing 1000g of the above-mentioned bulk drug (crude drug in step S1) in a fluidized bed, coating the bulk drug with 550g of the first isolation coating solution, and then drying until the moisture content is lower than 2%, to obtain Material A; wherein, the coating treatment conditions in this step are as follows: material temperature is 32°C, air inlet temperature is 60°C, air outlet te...
Embodiment 2
[0061] This embodiment provides a kind of aspirin composition, and its preparation method comprises the following steps:
[0062] S1. Aspirin is used as the raw material drug; wherein, 80% of the particle size of the raw material drug can be controlled between 80-100 mesh.
[0063] S2, 50g of polyvinylpyrrolidone (K30) was added to 500g of ethanol while stirring, and continued to stir until the solution was clear, then added 50g of talcum powder to prepare a suspension to obtain the first isolation coating solution, spare.
[0064] S3. Put 1000g of the above-mentioned crude drug in a fluidized bed, coat the crude drug with 600g of the first isolation coating solution, and then dry it until the water content is lower than 2%, to obtain material A; wherein, this step The conditions of the coating treatment are as follows: the material temperature is 30°C, the inlet air temperature is 50°C, the outlet air temperature is 25°C, and the air inlet volume is 0.5m 3 / min / kg, the liqu...
Embodiment 3
[0072] This embodiment provides a kind of aspirin composition, and its preparation method comprises the following steps:
[0073] S1. Aspirin is used as the raw material drug; wherein, 80% of the particle size of the raw material drug can be controlled between 80-100 mesh.
[0074] S2. Add 20g of hydroxypropyl methylcellulose into the mixed solvent of 280g of water and 300g of ethanol while stirring, and continue to stir until the solution is clear, then add 50g of silicon dioxide and 50g of talc Powders were mixed to prepare a suspension to obtain the first isolation coating liquid, which was set aside.
[0075] S3. Put 1000g of the above-mentioned crude drug in a fluidized bed, coat the crude drug with 648g of the first isolation coating solution, and then dry it until the water content is lower than 2%, to obtain material A; wherein, this step The conditions of the coating treatment are as follows: the material temperature is 35°C, the air inlet temperature is 65°C, the ai...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com