Bone repair material and preparation method thereof
A bone repair and material composition technology, applied in the field of medical biomaterials, can solve the problems of long curing time of CPC, slow degradation speed, loose artificial joints, etc., avoid the risk of secondary surgery, excellent in vitro degradation performance, and preparation method simple and easy effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] Formula: 6MgO+ Ca(H 2 PO 4 ) 2 + 4NaH 2 PO 4 +Calcium deficiency HA (20%)
[0104] The preparation method of bone cement composite bone repair material comprises the following steps:
[0105] (1) Dissolve calcium-deficient hydroxyapatite particles into 200 μmol / L deferoxamine solution, stir for 2 hours, and then centrifuge and dry to obtain the required drug-loaded calcium-deficient hydroxyapatite particles;
[0106] (2) According to the above formula, uniformly mix magnesium oxide, calcium dihydrogen phosphate, sodium dihydrogen phosphate and calcium-deficient hydroxyapatite to obtain bone cement powder;
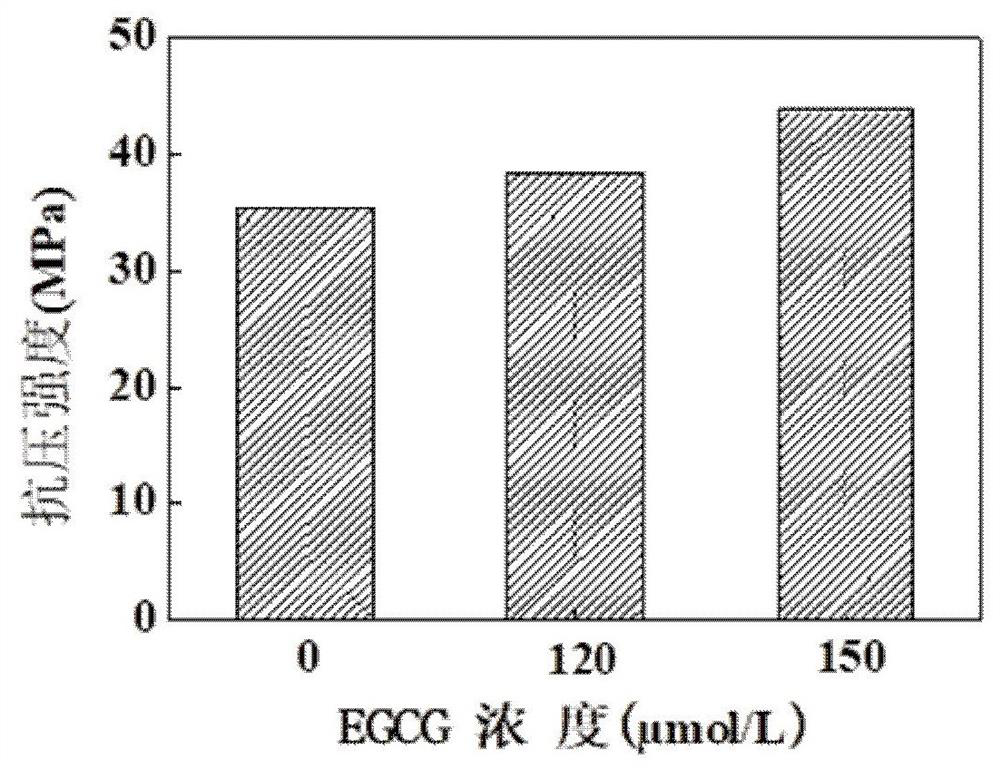
[0107] (3) Mix the bone cement powder and EGCG solidification solution (120 μmol / L) at a ratio of 0.55 g / 100 mg, and place them in a mold at 37°C and 100% humidity after solidification (setting time is 6-10 min). It can be cured for 72 h under ambient conditions.
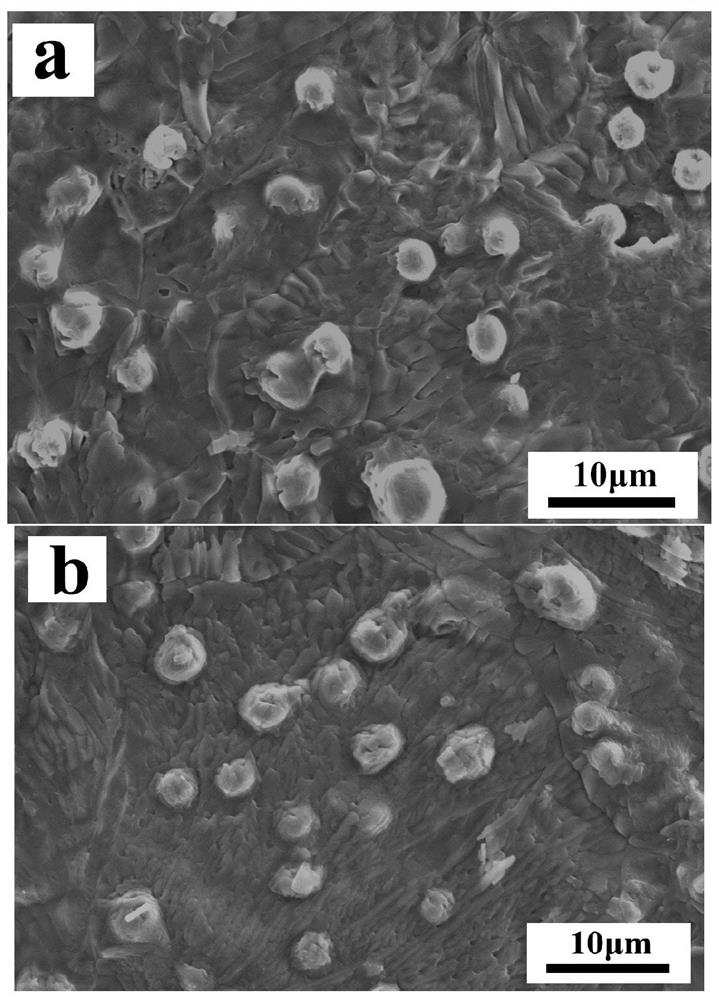
[0108] In bone cement composite bone repair materials, the particle size of calcium-deficient h...
Embodiment 2
[0110] Formula: 5MgO+ Ca(H 2 PO 4 ) 2 + 3NaH 2 PO 4 +Calcium deficiency HA (20%)
[0111] The preparation method of bone cement composite bone repair material comprises the following steps:
[0112] (1) Dissolve calcium-deficient hydroxyapatite particles into 200 μmol / L deferoxamine solution, stir for 2 hours, and then centrifuge and dry to obtain the required drug-loaded calcium-deficient hydroxyapatite particles;
[0113] (2) According to the above formula, uniformly mix magnesium oxide, calcium dihydrogen phosphate, sodium dihydrogen phosphate and calcium-deficient hydroxyapatite to obtain bone cement powder;
[0114] (3) Mix bone cement powder and EGCG solidification solution (120 μmol / L) at a ratio of 0.55 g / 120 mg, and place them in an environment of 37°C and 100% humidity after solidification in the mold (setting time is 6-10 minutes). It can be cured for 72 h.
[0115] In bone cement composite bone repair materials, the particle size of calcium-deficient hydroxya...
Embodiment 3
[0117] Formula: 3MgO+ Ca(H 2 PO4) 2 + NaH 2 PO 4 + Calcium-deficient HA (0%, 10% or 20%)
[0118] The preparation method of bone cement composite bone repair material comprises the following steps:
[0119] (1) According to the above formula, uniformly mix magnesium oxide, calcium dihydrogen phosphate, sodium dihydrogen phosphate and calcium-deficient hydroxyapatite to obtain bone cement powder;
[0120] (2) Evenly mix deferoxamine powder and bone cement powder;
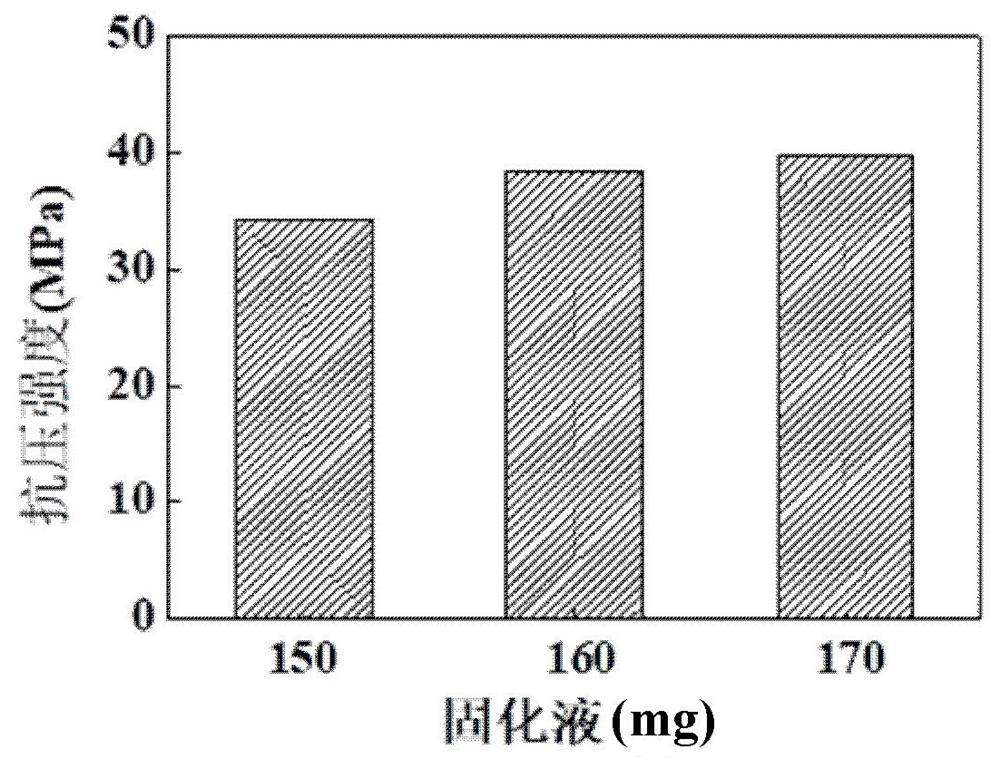
[0121] (3) Mix deferoxamine-containing bone cement powder with EGCG solidification solution (120 μmol / L) at a ratio of 0.55 g / 150 mg, and put it in the mold at 37°C after solidification (setting time is 6-10 min). It can be cured for 72 h under the environment of 100% humidity.
[0122] In bone cement composite bone repair materials, the particle size of calcium-deficient hydroxyapatite is 3-5 µm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com