High-activity catalyst used for hydrogen production by dehydrogenation of organic hydrogen storage compound and reduced in noble metal consumption, and preparation method thereof
The technology of catalyst and compound is applied in the field of dehydrogenation hydrogen production catalyst of highly active organic hydrogen storage compound with reduced amount of precious metal and its preparation field, which can solve the problems of high price and low dehydrogenation activity of dehydrogenation catalyst.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
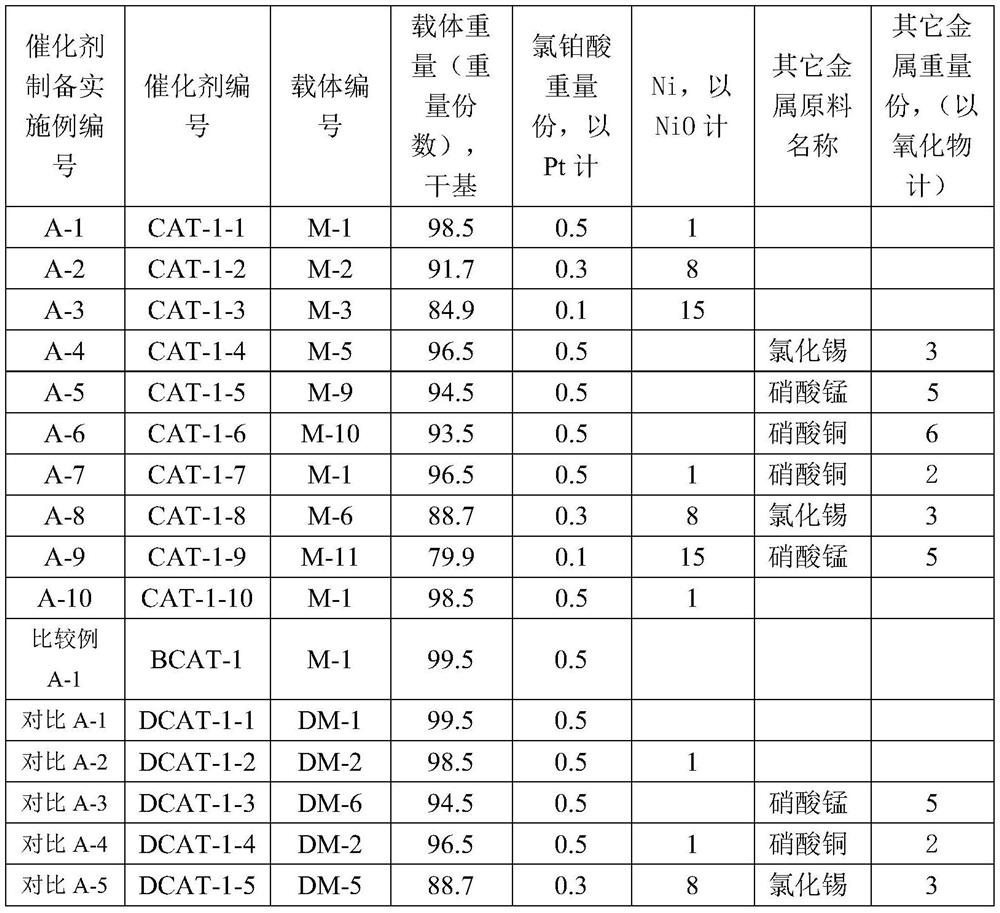
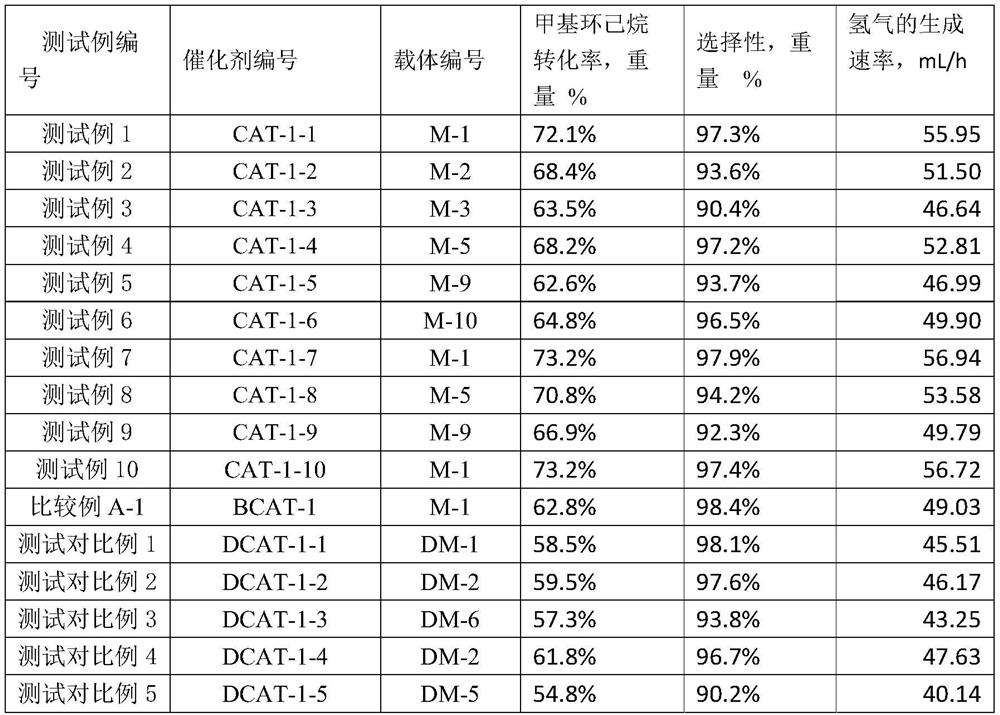
[0056] In the catalyst preparation method provided by the present invention, in step (1), the airflow of the modified metal oxide precursor carried by the gas is contacted with the alumina substrate, and the temperature of the contact is preferably 15-350° C., for example, 15-300° C. or 15-300° C. 100°C or 15-200°C or 18-60°C or 15-40°C. The temperature of the gas is room temperature-350°C, for example, room temperature-300°C or 15-300°C. The room temperature is, for example, 15-40° C., and the contact pressure can be 0.05-5 atm, for example, 1-3 atm. When the modified metal precursor on the alumina substrate reaches a preset loading capacity, the contact with the modified metal oxide precursor carried by the gas is stopped to obtain an alumina substrate loaded with the modified metal oxide precursor. The time that the alumina substrate is in contact with the gas-entrained stream of the modified metal oxide precursor is referred to as the loading time. In one embodiment, the...
Embodiment 1
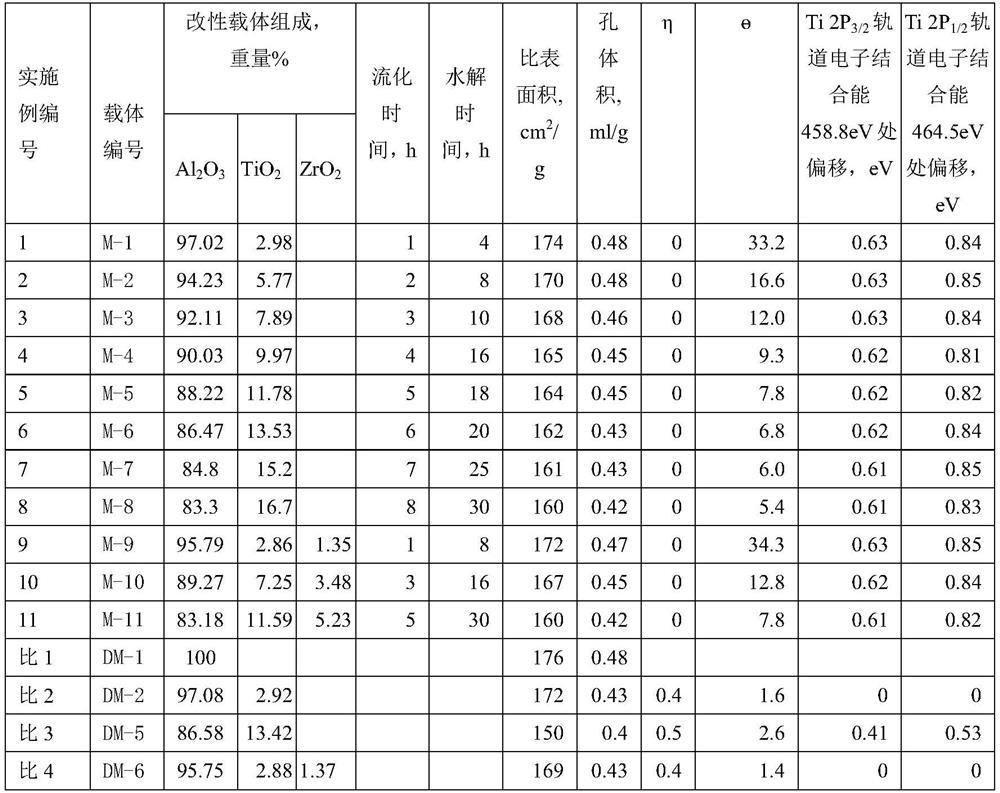
[0074] Calcining SB powder at 500℃ for 4h to obtain γ-Al 2 o 3 , the γ-Al 2 o 3 The specific surface area is 176m 2 / g, the pore volume is 0.48ml / g.
[0075] Take the above γ-Al 2 o 3 500g is placed in the fluidized reactor (the diameter of the reactor is 10cm, and the height is 40cm), titanium tetrachloride is placed in a 20°C constant temperature bath, nitrogen (temperature 25°C) passes through the titanium tetrachloride at a flow rate of 10L / min and then Enter the fluidized reactor from the bottom of the fluidized reactor, and after fluidizing for 1 hour, stop passing the nitrogen gas through the titanium tetrachloride bath; The ionized water enters the fluidized reactor from the bottom of the reactor, and is fluidized for 4 hours for hydrolysis to obtain a hydrolyzed carrier. The hydrolyzed carrier was calcined in an air atmosphere at 550° C. for 4 hours to obtain a carrier composition, which was named M-1. The properties of the carrier are shown in Table 1.
Embodiment 2~ Embodiment 8
[0077] The preparation method is the same as carrier preparation example 1, the difference lies in the time for nitrogen to carry titanium tetrachloride into the fluidized bed, and the hydrolysis time for nitrogen to pass through deionized water. The specific values and carrier properties are shown in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com