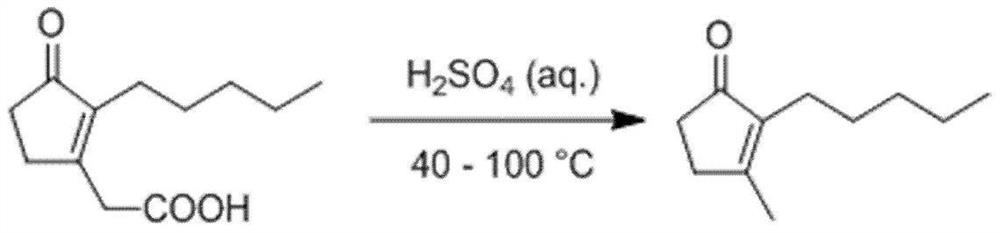
Method for synthesizing dihydrojasmones
A technology of dihydrojasmonone and sulfuric acid solution is applied in chemical instruments and methods, preparation of organic compounds, separation/purification of carbonyl compounds, etc. The effect of simple treatment, reaction conditions, and simple synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] S1. Add 200 g concentration of 10% sulfuric acid aqueous solution to the 2000mL reaction kettle equipped with mechanical stirring and reflux condenser, stir evenly, and heat to reflux;
[0026] S2. dripping 938g content to the system is 92% 3-oxo-2-pentyl-1-cyclopentene-1-acetic acid (recorded by gas chromatography quantitative analysis), the rate of addition is 14g / min, and then React at 40°C under reflux for 6 hours. After the reaction, let stand to separate the layers, and separate the aqueous phase 1 to obtain the organic phase 1;
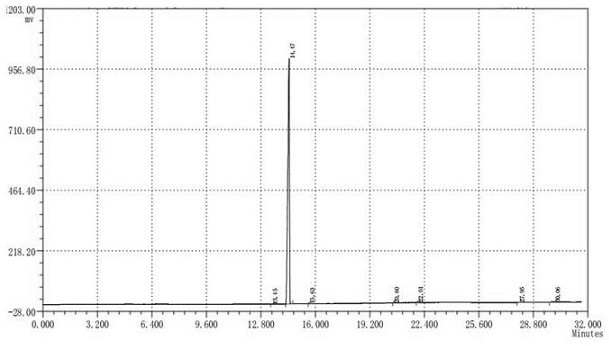
[0027] S3. Add 375g of 10% sodium sulfate aqueous solution to the organic phase 1, stir for 10min, separate the aqueous phase 2 to obtain the organic phase 2 after standing for stratification, the organic phase 2 is vacuumed at 0.5mmHg, the kettle temperature is 100°C, and the steam temperature is 88°C Under reduced pressure distillation, the fractions were collected to obtain 560 g of dihydrojasmone with a content of 99.6%. The yield i...
Embodiment 2
[0030] S1. Adding 200 g concentration of 15% sulfuric acid aqueous solution in the 2000mL reaction kettle equipped with mechanical stirring and reflux condenser, stir evenly, and heat to reflux;
[0031] S2. adding 800g content dropwise to the system is 92% 3-oxo-2-pentyl-1-cyclopentene-1-acetic acid (recorded by gas chromatography quantitative analysis), the rate of addition is 16g / min, and then React at 80°C under reflux for 5 hours. After the reaction, stand and separate the layers, and separate the aqueous phase 1 to obtain the organic phase 1;
[0032] S3. Add 400g of 10% sodium sulfate aqueous solution to the organic phase 1, stir for 20min, separate the aqueous phase 2 to obtain the organic phase 2 after standing for stratification, and the organic phase 2 is vacuumed at 0.5mmHg, the kettle temperature is 115°C, and the steam temperature is 89°C Under reduced pressure distillation, the fractions were collected to obtain 485 g of dihydrojasmone with a content of 99.6%. ...
Embodiment 3
[0035] S1. In the 2000mL reaction kettle that mechanical stirring and reflux condenser are housed, add 200g concentration and be the sulfuric acid aqueous solution of 30%, stir evenly, be heated to reflux;
[0036] S2. dropwise adding 938g content to the system is 92% 3-oxo-2-pentyl-1-cyclopentene-1-acetic acid (recorded by gas chromatography quantitative analysis), the rate of addition is 17g / min, and then React at 100°C under reflux for 4 hours. After the reaction, let stand to separate and separate the water phase 1 to obtain the organic phase 1;
[0037] S3. Add 562g of 10% aqueous sodium sulfate solution to the organic phase 1, stir for 30min, separate the aqueous phase 2 to obtain the organic phase 2 after standing for stratification, and the organic phase 2 is vacuumed at 0.5mmHg, the kettle temperature is 115°C, and the steam temperature is 90°C Under reduced pressure distillation, the fractions were collected to obtain 568g of dihydrojasmone with a content of 99.5%. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com