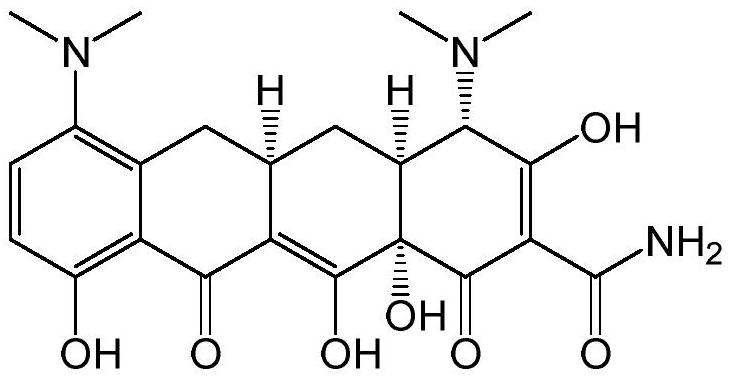
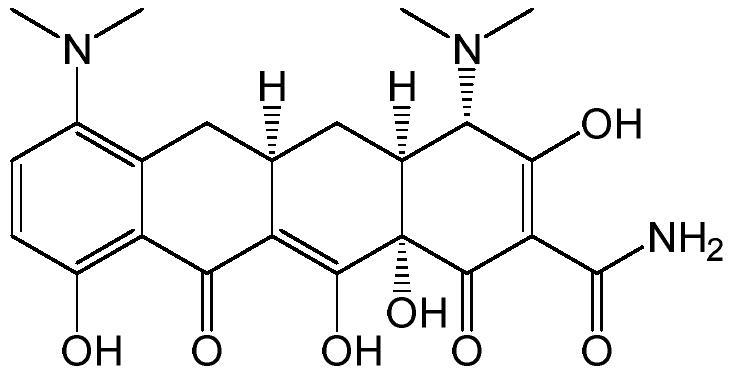
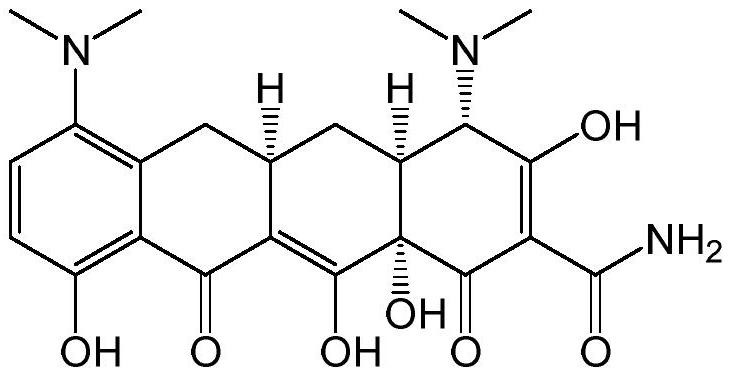
Preparation method of minocycline
A technology of minocycline and sancycline, which is applied in the field of preparation of minocycline, can solve problems such as difficult purification, and achieve the effect of simple purification and simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Embodiment 1 Sancycline 6-protection of ethoxycarbonyl
[0036] The structural formula is as follows:
[0037]
[0038] Sancycline (2 mmol), dichloromethane (5 mL) and triethylamine (3 mmol) were added in a 50 mL flask. The mixture was cooled to 0°C, and ethyl chloroformate (2.2 mmol) was slowly added dropwise, keeping the temperature not exceeding 5°C. The reaction was monitored by TLC, and the conversion was complete within 1 hour. Add 10 ml of water and separate the layers. The organic phase was concentrated to dryness and used directly in the next reaction without purification. ESI-MS m / z: 487.2 (M+H+).
Embodiment 2
[0039] Embodiment 2 Sancycline 6-protection of acetyl group
[0040] The structural formula is as follows:
[0041]
[0042] Shancycline (2 mmol), acetic anhydride (6 mmol) and pyridine (6 mmol) were added to 50 ml of dichloromethane and refluxed for not less than 5 hours. After the reaction is complete, cool down to room temperature, adjust to neutrality with saturated aqueous sodium bicarbonate solution, wash the organic phase with 12% brine, dry over anhydrous sodium sulfate, filter, concentrate to dryness, and directly use in the next reaction without purification . ESI-MS m / z: 457.3 (M+H+).
Embodiment 3
[0043] Embodiment 3 Sancycline 6-protection of benzoyl
[0044] The structural formula is as follows:
[0045]
[0046] Sancycline (2mmol), sodium carbonate (5g) and benzoyl chloride (2.5mmol) were added to 50ml of methanol and stirred at 25-35°C for 4 hours. TLC showed that the reaction was complete, 100 ml of purified water was added, adjusted to neutrality with saturated aqueous sodium bicarbonate solution, the organic phase was washed with 12% brine, dried over anhydrous sodium sulfate, filtered, concentrated to dryness, and used directly for Next reaction. ESI-MS m / z: 518.9 (M+H+).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com