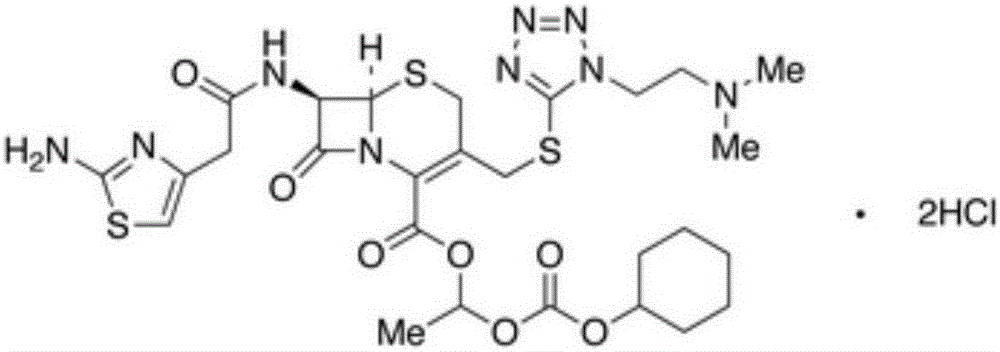
Synthesis method of cefotiam hexetil process impurity
A kind of cefotiam pivoxil and technology of synthesis method, applied in the field of medicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
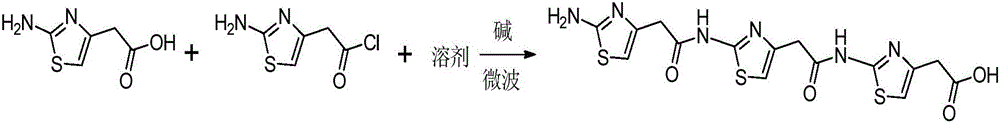
[0021] Add 15.8g (0.1mol) of 2-amino-4-thiazoleacetic acid, 44g (0.25mol) of 2-amino-4-thiazole acetyl chloride, and 268ml of water in sequence in the reaction flask, add 35ml of triethylamine, and put the reaction flask into In a microwave reactor, react at 125°C for 20 minutes, adjust the pH to 3.0 with 1N concentrated hydrochloric acid, precipitate a light yellow solid, filter, and wash the filter residue with water and acetone in sequence to obtain 39.4 g of the light yellow target product, with a yield of 90% and a liquid phase purity of 99.6 %. 1 H-NMR (DMSO-d 6 ):6.98(s,1H),6.95(s,2H),6.73(s,1H),6.31(s,1H),3.92(s,2H),3.54(s,2H),3.32(s,2H) . 13 C-NMR (DMSO-d 6 ):172.7,168.8,168.7,168.6,158.0,157.8,148.4,145.3,145.1,110.5,106.2,103.4,41.7,38.6,38.3.LC-MS[M+H] + :439.0.
[0022] HPLC detection conditions are as follows:
[0023] Column: CAPCELL PAK ACR-C18 liquid chromatography column, inner diameter 4.6mm, length 25cm, particle diameter 5μm. Column temperature: 35°...
Embodiment 2
[0025] Add 15.8g (0.1mol) of 2-amino-4-thiazoleacetic acid, 44g (0.25mol) of 2-amino-4-thiazole acetyl chloride, and 268ml of water in sequence in the reaction flask, add 35ml of triethylamine, and put the reaction flask into In an oil bath, react at 125°C for 20 minutes, adjust the pH to 3.0 with 1N concentrated hydrochloric acid, precipitate a light yellow solid, filter, and wash the filter residue with water and acetone in sequence to obtain 0.9 g of the light yellow target product, with a yield of 2% and a liquid phase purity of 92.5% .
Embodiment 3
[0027] Add 15.8g (0.1mol) of 2-amino-4-thiazoleacetic acid, 44g (0.25mol) of 2-amino-4-thiazole acetyl chloride, and 268ml of water in sequence in the reaction flask, add 35ml of triethylamine, and put the reaction flask into In an oil bath, react at 125°C for 12 hours, adjust the pH to 3.0 with 1N concentrated hydrochloric acid, precipitate a light yellow solid, filter, and wash the filter residue with water and acetone successively to obtain 8 g of the light yellow target product, with a yield of 22.5% and a liquid phase purity of 92.7%.
[0028] Through the comparison of Example 1, Example 2, and Example 3, it is found that microwave-assisted catalysis significantly improves the reaction rate and yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com