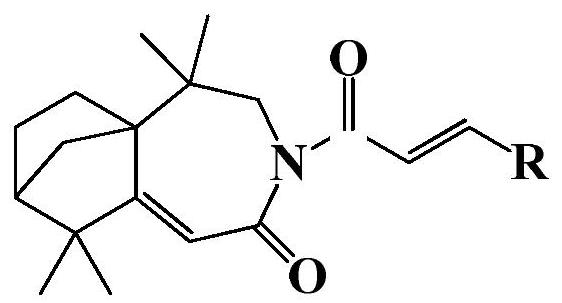
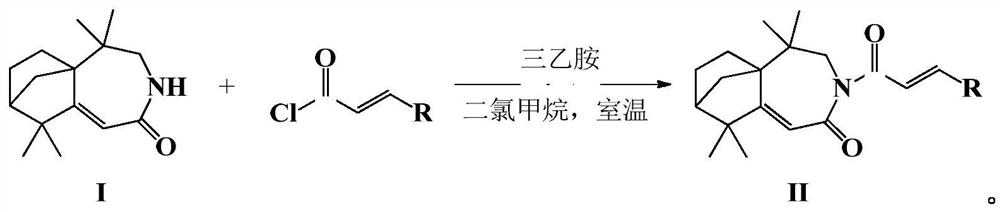
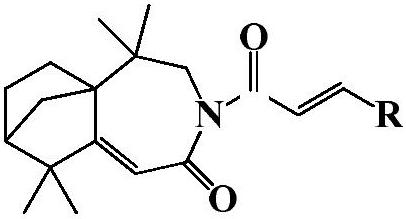
Isolongifolenone caprolactam derivative as well as preparation method and application thereof
A technology of phyllenone and caprolactam, which is applied in the fields of medicinal chemistry and pharmacotherapeutics, can solve the problems of accumulation of toxicity and low anti-tumor activity, and achieve the effect of abundant sources and favorable industrial production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] 3-(4′-fluoro)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[d ] Preparation of azepine-2 (3H)-ketone (compound 1):
[0024]
[0025] Add 5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[d]azepine sequentially into a 50mL one-necked flask In-2(3H)-ketone (0.5mmol), triethylamine (5mmol) and anhydrous dichloromethane (20mL), add p-fluorocinnamoyl chloride (0.5mmol) dropwise under ice bath, and react at room temperature for 4h after the dropwise addition , TLC detected that the reaction was complete, added water, extracted with dichloromethane, combined, concentrated, and purified by column chromatography (petroleum ether:ethyl acetate=4:1) to obtain a white solid powder with a yield of 74.5%. 1 HNMR (600MHz, CDCl 3 )δ: 7.64(d, J=15.5Hz, 1H), 7.54(dd, J=8.6, 5.5Hz, 2H), 7.21(d, J=15.6Hz, 1H), 7.04(t, J=8.6Hz, 2H), 5.74(s, 1H), 4.11(d, J=14.4Hz, 1H), 3.56(d, J=14.4Hz, 1H), 1.97(s, 1H), 1.89-1.91(m, 1H), 1.78-1.83(m, 1H), 1.72(d, J=7....
Embodiment 2
[0027] 3-(4′-methyl)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[ d] Preparation of azepine-2(3H)-one (compound 2):
[0028]
[0029] The preparation method is the same as in Example 1, replacing p-fluorocinnamoyl chloride with p-methylcinnamoyl chloride, and the product 3-(4′-methyl)cinnamoyl-5,5,9,9-tetramethyl-4,5,6, 7,8,9-hexahydro-5a,8-methanobenzo[d]azepin-2(3H)-one is a white solid powder with a yield of 73.8%. 1 H NMR (600MHz, CDCl 3 )δ: 7.68(d, J=15.5Hz, 1H), 7.45(d, J=8.1Hz, 2H), 7.25(d, J=15.5Hz, 1H), 7.16(d, J=7.9Hz, 2H) , 5.74(s, 1H), 4.10(d, J=14.5Hz, 1H), 3.56(d, J=14.5Hz, 1H), 2.35(s, 3H), 1.96(s, 1H), 1.88-1.93( m, 1H), 1.77-1.82(m, 1H), 1.71(d, J=10.2, 1H), 1.51-1.57(m, 1H), 1.39(d, J=10.1Hz, 1H), 1.27-1.32( m, 1H), 1.16(s, 3H), 1.09(s, 3H), 1.05(s, 3H), 0.96(s, 3H); 13 C NMR (151MHz, CDCl 3 )δ:176.42,170.11,170.02,142.90,140.15,132.53,129.44,128.25,120.99,114.25,63.70,49.14,46.07,45.47,37.68,34.34,28.90,28.76,25.37,24.82,24....
Embodiment 3
[0031] 3-(4′-chloro)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7,8,9-hexahydro-5a,8-methanobenzo[d ] Preparation of azepine-2 (3H)-ketone (compound 3):
[0032]
[0033] The preparation method is the same as in Example 1, replacing p-fluorocinnamoyl chloride with p-chlorocinnamoyl chloride, and the product 3-(4'-chloro)cinnamoyl-5,5,9,9-tetramethyl-4,5,6,7, 8,9-hexahydro-5a,8-methanobenzo[d]azepin-2(3H)-one is a white solid powder with a yield of 77.8%. 1 H NMR (600MHz, CDCl 3 )δ: 7.64(d, J=15.6Hz, 1H), 7.51(d, J=8.5Hz, 2H), 7.34(d, J=8.5Hz, 2H), 7.27(d, J=15.7Hz, 1H) , 5.76(s, 1H), 4.14(d, J=14.5Hz, 1H), 3.58(d, J=14.4Hz, 1H), 1.98(m, 1H), 1.91-1.95(m, 1H), 1.80- 1.84(m, 1H), 1.74(d, J=10.1, 1H), 1.60-1.53(m, 1H), 1.43(d, J=10.2, 1H), 1.30-1.35(m, 1H), 1.19(s , 3H), 1.12(s, 3H), 1.08(s, 3H), 0.98(s, 3H); 13 C NMR (151MHz, CDCl 3 )δ: 176.86, 170.04, 169.71, 141.04, 135.58, 133.81, 129.37, 128.96, 122.68, 114.08, 63.75, 49.12, 46.06, 45.54, 37.67, 34.31, 28.87, 28.79, 24.2.2
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com