Compound and preparation method thereof, fluorescent probe and antitumor drug
A technology of fluorescent probes and compounds, applied in the field of organic synthesis, can solve the problems of unstable non-biodegradability and long-term toxicity, and achieve the effect of photothermal therapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
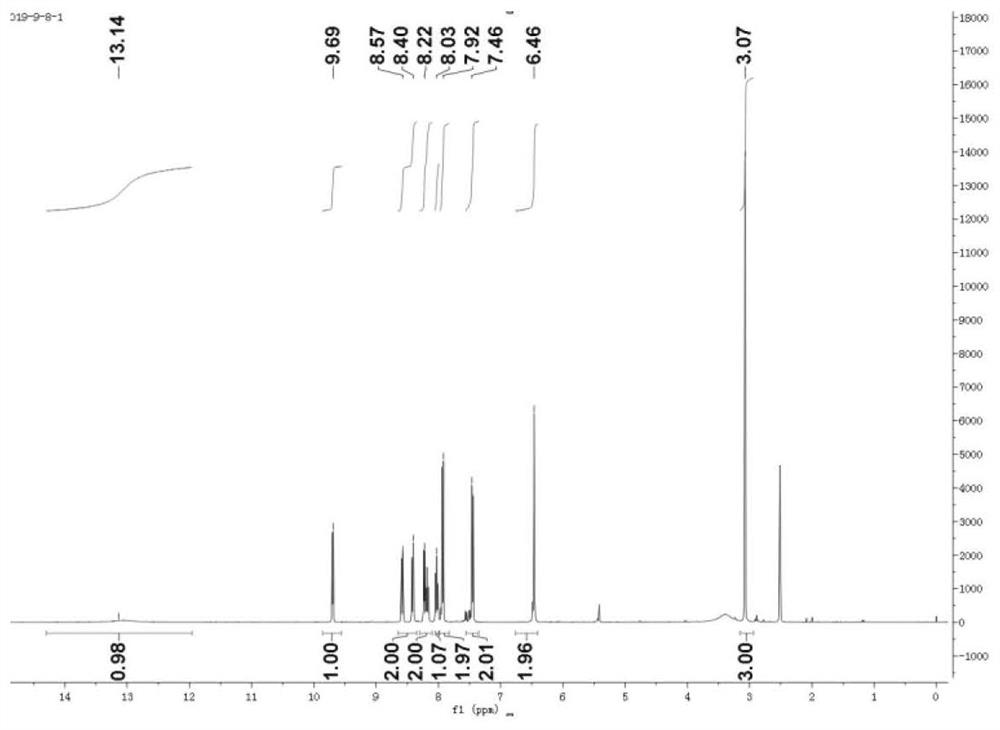
[0049] Present embodiment is the synthesis of compound (TPHP) shown in formula (VI)
[0050]
[0051]
[0052] (1) Accurately weigh 0.143g of compound 1 and 0.214g of compound 2 and mix, add 10ml of anhydrous acetonitrile as solvent, react at 70°C for 24h to obtain compound 3 (compound of formula II).
[0053] (2) Weigh 0.181g of compound 4 and 0.214g of 1,4-dibromobutane, add 10ml of anhydrous DMF after mixing as a reaction solvent, and 200ul of triethylamine as a catalyst, react at room temperature for 12h to obtain compound 5 ( compound of formula III).
[0054] (3) Mix 0.397 g of compound 5 and 0.358 g of compound 3, add 200 μl of triethylamine as a solvent, and stir at 300 rpm for 24 hours at room temperature to obtain compound 6 (compound of formula IV).
[0055] (4) Mix 0.626g of compound 6 with 0.262g of triphenylphosphine, add 10ml of anhydrous acetonitrile as a solvent, weigh 100mg of sodium carbonate as a catalyst, and react at 120°C for 12h to obtain compoun...
Embodiment 2
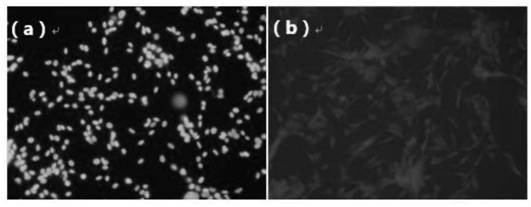
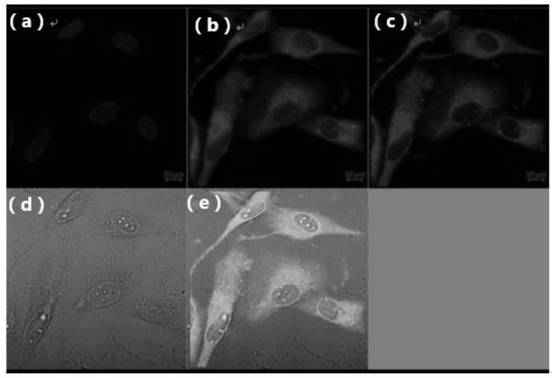
[0060] The present embodiment is the optical property of TPHP and photostability determination
[0061] In order to investigate the photothermal performance, different concentrations of TP-PPh 3 (Formula V compound) and TPHP nano micelles were exposed to 808nm (1.0w / cm 2 ) in the laser for 5min. At predetermined time points, the regional maximum temperature of the solution was monitored using an infrared thermal imaging camera, with phosphate buffered saline as a negative control.
[0062] TP-PPh 3 (Such as Figure 4 shown) and TPHP have good photothermal properties, and with the increase of laser density and TP-PPh 3 With the increase of the concentration of TPHP, the temperature of the solution continued to rise, indicating that TP-PPh 3 and TPHP have good photostability, so TP-PPh 3 And TPHP converts the absorbed light energy into heat energy, thereby ablate tumor and realize photothermal therapy.
Embodiment 3
[0064] This embodiment establishes the subcutaneous model for bearing U87 mice
[0065] Prepare 6-8 week-old Balb / c nude mice, digest U87 cells in logarithmic growth phase, resuspend, and count. For the inoculation of mice with subcutaneous tumors, prepare 5 × 10 6 Cells (100 μl) were injected subcutaneously in the lower right lower back of the mouse. The size of the subcutaneous tumor in the mouse to be observed grows to 60mm 3 Around , the mice were divided into TP-PPh 3 Group and TPHP group, with 5 mice in each group, for subsequent imaging experiments.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com