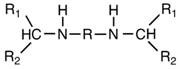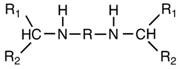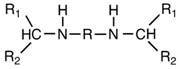Preparation method of aliphatic diamine with high steric hindrance effect
An aliphatic binary, high steric hindrance technology, which is applied in the fields of special chemicals and fine chemicals, can solve the problems of low purity and high functionality of curing agents, and achieve good yellowing resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Embodiment 1: A kind of preparation method of the aliphatic dibasic secondary amine of high steric hindrance effect
[0026] The object of the present invention is to provide an environment-friendly preparation method of linear / branched aliphatic secondary secondary amines with high product yield, high purity, convenient operation, non-toxic and pollution-free low reactivity. The target product can impart good flexibility to the product while significantly reducing the reactivity.
[0027]This secondary amine has two substituted alkyl groups on the α-carbon of the amino group, that is, the substituent on the N atom belongs to the secondary carbon-substituted alkyl group, and the steric hindrance effect is obvious, which effectively reduces the reactivity of the secondary amino group. And can give the product good flexibility. However, the synthesis of aliphatic dibasic secondary amines containing large alkyl substituents on the amino group is difficult.
[0028] The s...
Embodiment 2
[0048] Embodiment 2: A kind of preparation method of the aliphatic secondary amine of high steric hindrance effect
[0049] Add 88.2 g (1.0 mol) of 1,4-butylene diamine, 426.7 g (3.0 mol) of diisobutyl ketone and 0.9 g of platinum carbon catalyst into a stainless steel high-pressure reaction vessel, close the feeding port, and replace the reaction vessel with nitrogen The air was replaced with hydrogen for 3 times and the nitrogen was replaced with hydrogen for 3 times, then the temperature was raised, the temperature was controlled at 60°C, the pressure of hydrogen was not higher than 6.0 MPa, and the reaction was carried out for 10 hours under these conditions, and then the temperature was gradually increased, and the maximum reaction temperature was controlled within 92°C , after a total reaction of 25 hours, the temperature was lowered to 28°C, the hydrogen was vented, and the residual hydrogen was replaced with nitrogen, and the pressure was returned to normal. The reacti...
Embodiment 3
[0051] Embodiment 3: A kind of preparation method of the aliphatic dibasic secondary amine of high steric hindrance effect
[0052] Put 102.8g (1.0 mol) of 1,5-pentanediamine, 274.1g (2.4 mol) of 2,4-dimethyl-3-pentanone and 5g of palladium-carbon catalyst into a stainless steel high-pressure reaction vessel, and replace the reaction with nitrogen Air in container 3 times. Replace the nitrogen with hydrogen for 3 times, raise the temperature, control the hydrogen pressure to 3.0~3.5 MPa, the initial reaction temperature is 60°C, gradually increase the temperature as the reaction progresses, the maximum reaction temperature is controlled within 95°C, after the hydrogenation reaction for 20 hours , lower the temperature, vent the hydrogen, replace the residual hydrogen with nitrogen, and return to normal pressure. The reaction product is filtered, and the filter residue is used as a catalyst for recycling. First distill off excess 2,4-dimethyl-3-pentanone, by-product water and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com