Method for preparing high-purity fatty acid derivative
A technology of fatty acid derivatives and long-chain fatty acids, which is applied in the field of biomedicine and can solve problems such as difficulties in the preparation of fatty acid derivatives, environmental pollution by by-products, and reduced yields.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
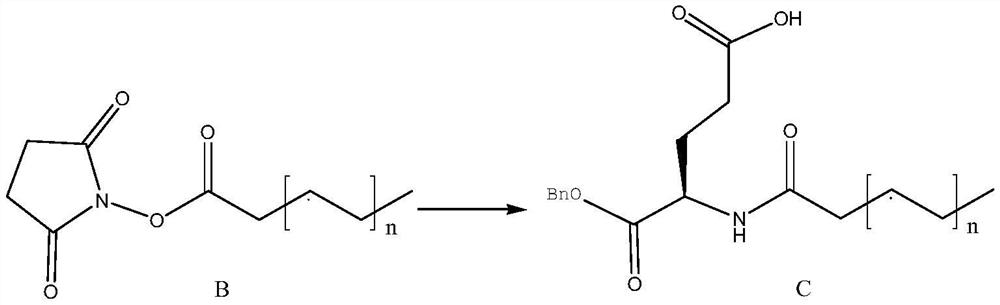
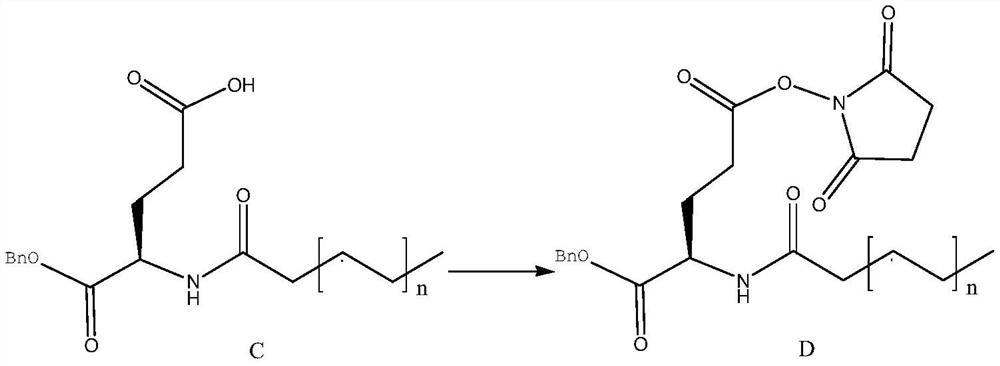
Examples
Embodiment 1
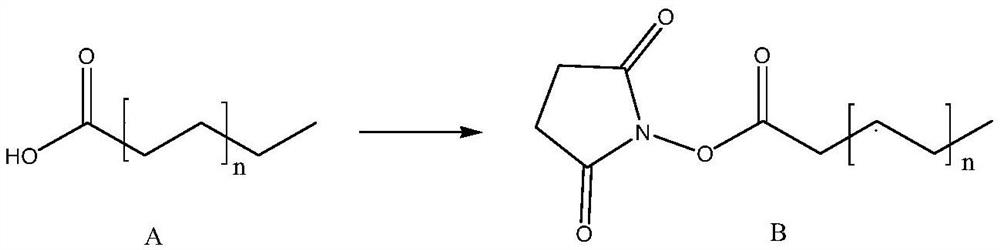
[0064] 1. Preparation of succinimidyl palmitate
[0065]
[0066] Palmitic acid (100g, 390.0mmol) was suspended in N,N-dimethylformamide (2000ml), and N-hydroxysuccinimide (49.3g, 429.0mmol) and dicyclohexyl carbon were added at -10°C Diimine (88.5 g, 429.0 mmol), continued to react at -10°C for 2 hours, then returned to room temperature (25°C) and stirred overnight. Suction filtration, the filtrate was concentrated under reduced pressure, 2000 mL of dichloromethane was added to the concentrated residue, washed successively with 1000 mL of saturated sodium bicarbonate, 3 times with 1000 mL of water, 1000 mL of saturated brine, dried over anhydrous sodium sulfate, filtered, and the filtrate was Dichloromethane was removed by pressure distillation to obtain a white solid residue, to which n-heptane was added for recrystallization, the filter cake was collected by filtration, the filter cake was washed with n-heptane (300ml), and the filter cake (being succinimide palmitate) w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com