A kit for predicting efficacy of immune checkpoint inhibitor on cancer patients
A technology of immune checkpoint and kit, applied in the field of immunotherapy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
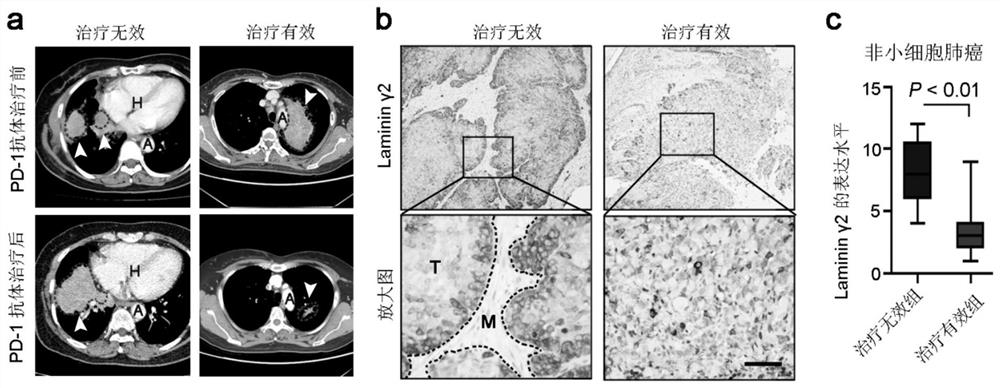
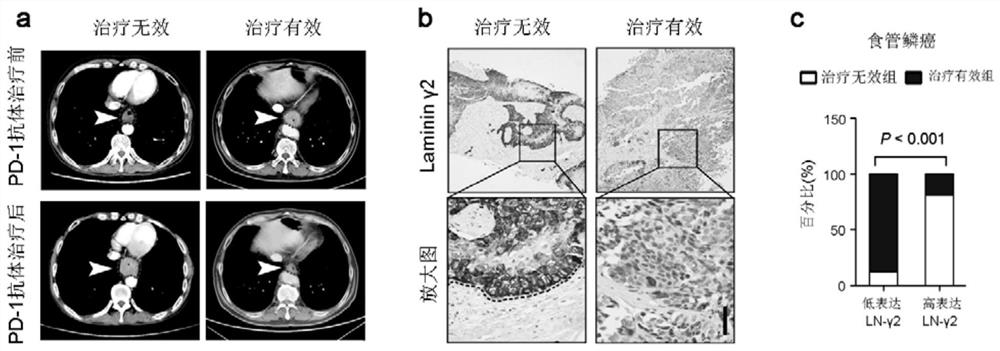
[0029] Example 1 The expression level of Laminin-γ2 is closely related to the efficacy of PD-1 antibody drugs
[0030] 35 patients with non-small cell lung cancer who were effectively treated with PD-1 monoclonal antibody, 36 patients with non-small cell lung cancer who were ineffective with PD-1 monoclonal antibody, and 26 patients with PD-1 Immunohistochemical staining was used to detect the expression of laminin Laminin-γ2 in the tumor tissues of 1 patient with effective monoclonal antibody therapy and 17 patients with esophageal squamous cell carcinoma PD-1 monoclonal antibody therapy ineffective. Immunostaining scoring method: staining intensity: negative = 0 points, weak expression = 1 point, moderate expression = 2 points, strong expression = 3 points; positive staining area: <25% = 1 point, 25%-50% = 2 points , 50%-75%=3 points, ≥75%=4 points; total score=staining intensity×staining area. A total score of less than 3 is considered as low expression; a total score of ≥...
Embodiment 2
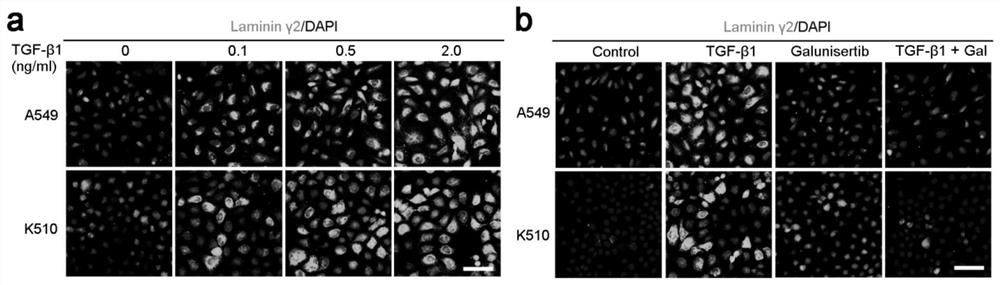
[0032] Example 2 TGF-β1 promotes the expression of laminin-γ2 in tumor cells
[0033] (1) The effect of TGF-β1 concentration on the expression of laminin-γ2 in tumor cells.
[0034] Lung cancer cells (A549, purchased from ATCC, #CCL-185) and esophageal squamous cell carcinoma cells (K510, purchased from DSMZ, #ACC 374) were respectively digested with trypsin and planted on cell slides. After the cells adhered to the wall, replace The medium added with TGF-β1 recombinant protein (TGF-β1 recombinant protein concentrations were 0, 0.1, 0.5, 2.0 ng / ml), TGF-β1 recombinant protein was purchased from R&D Systems, #P01137. After 24 hours of treatment, the expression level of laminin-γ2 in cancer cells was detected by immunofluorescence staining.
[0035] The results show that: if image 3As shown in a, immunofluorescence staining shows that TGF-β1 recombinant protein can significantly stimulate the expression of laminin Laminin-γ2 in lung cancer cells (A549) and esophageal squamous...
Embodiment 3
[0043] Embodiment 3 combination therapy inhibits tumor growth
[0044] (1) Establishment of mouse subcutaneous xenograft tumor model
[0045] Twenty-six C57BL / 6 mice with sound immunity were subcutaneously injected with mouse lung cancer cells LLC (2×10 5 / only) to establish a subcutaneous xenograft tumor model, and combined therapy was carried out three weeks later.
[0046] (2) Combined therapy
[0047] Mouse PD-1 antibody was purchased from BioXcell (#BE0146), TGF-β1 receptor inhibitor Galunisertib was purchased from Selleck (#S2230), anticancer drug cisplatin was purchased from Selleck (#S1166), paclitaxel was purchased from Selleck (#S1150 ).
[0048] The subcutaneous xenograft tumor model mice obtained in step (1) were divided into 4 groups, including 5 mice in the control group and 7 mice in each drug treatment group:
[0049] Control group: injection of the same amount of sodium carboxymethylcellulose (1% CMC-Na);
[0050] Gal+anti-PD-1: Galunisertib (gastric admi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com