Preparation method of 2-methyl-1,3-pentadiene
A technology of pentadiene and methyl group, applied in the field of organic synthesis, can solve the problems such as restricting the production cost and market scale of privet aldehyde, difficult to achieve recovery and application of catalysts, poor selectivity of target products, etc., achieves superior catalytic performance, and avoids equipment corrosion. , the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
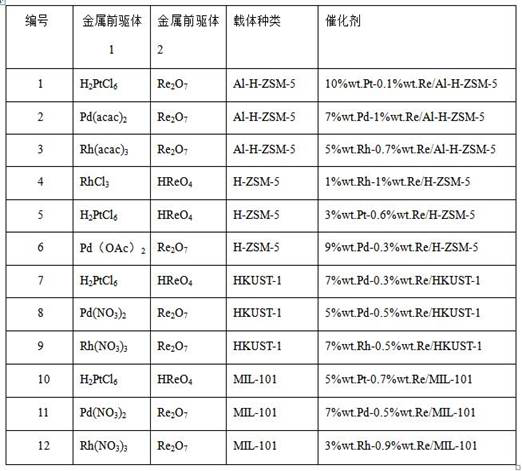
[0051] Weigh 100g of the carrier Al-H-ZSM-5, put it into a muffle furnace, bake it at 200°C for 4 hours, and let it cool naturally for later use.
[0052] Weigh 10g of Al-H-ZSM-5, add it to 60mL deionized water, slowly add 5mL of 0.266g / mL H 2 PtCl 6 The solution was stirred at 50°C for 8 hours, dried in an oven at 70°C for 12 hours, and calcined at 600°C in air for 2 hours. Finally, 5%wt.Pt / Al-H-ZSM-5 catalyst can be obtained by sodium borohydride reduction for 3 hours at 50℃.
[0053] Using the same impregnation method as above, changing the types and ratios of active components, carriers or catalyst aids to prepare catalysts loaded with other active components or catalyst aids. In addition, the catalysts supported by HKUST-1, MIL-100, MIL-101, MIL-125, MOF-901, and Uio-66 were adsorbed and dried in an oven at 70°C for 24 hours, and then directly reduced. The results are shown in Table 1. All the other operations are the same as in Example 1, see Table 1 for details.
...
Embodiment 2
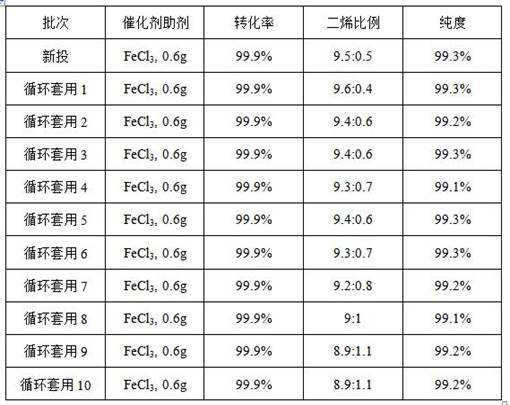
[0058] 5%wt. Pt / Al-H-ZSM-5 catalyzes the dehydration reaction of 2-methyl-2,4-pentanediol, as follows: In a 250mL three-necked flask, add 100g of 2-methyl-2,4-pentanediol Alcohol, 5g5%wt.Pt / Al-H-ZSM-5, 0.6g anhydrous ferric chloride, start stirring at 400 rpm, and heat to 130°C. Total reflux for 10 minutes, the temperature at the top of the tower is 73°C~75°C, and the extraction starts, and the reflux ratio is 5. After cumulative reaction rectification for 3 hours, the temperature was lowered to stop the reaction. The collected product was separated from water and dried and weighed 66.71g, with a purity of 99.2% and a diene yield of 95.3%. (The molar ratio of 2-methyl-1,3-pentadiene and 4-methyl-1,3-pentadiene in the product detected by gas chromatography is 9.3:0.7.) The catalyst was obtained by filtering the liquid in the tower kettle, and recovered Then continue to apply to the next batch of reactions.
Embodiment 3-10
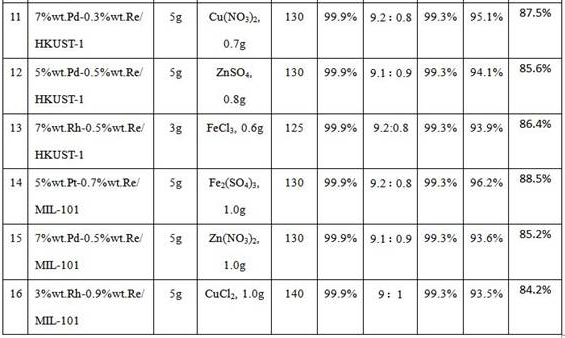
[0060] Performance comparison of different catalysts for the dehydration of 2-methyl-2,4-pentanediol to 2-methyl-1,3-pentadiene and 4-methyl-1,3-pentadiene . Except that the reaction temperature is slightly adjusted, other operations are the same as in Example 2. The results are shown in Table 2.
[0061] Table 2 The results of the investigation of the catalytic performance of different catalysts
[0062]
[0063] Note: The yield in Table 2 is the sum of the yields of 2-methyl-1,3-pentadiene and 4-methyl-1,3-pentadiene.
[0064] It can be seen from the results in Table 2 that the catalysts with better catalytic effects are 5%wt.Pt / Al-H-ZSM-5, 5%wt.Pd / H-ZSM-5, 5%wt.Re / HKUST- 1. 3%wt.Pd / MIL-101, 3%wt.Pt / MIL-100.
[0065] The total yield is multiplied by the proportion of 2-methyl-1,3-pentadiene, which is the yield of 2-methyl-1,3-pentadiene.
PUM
| Property | Measurement | Unit |
|---|---|---|
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com