Pichia pastoris for efficiently expressing recombinant porcine alpha1 interferon through auxiliary secretion
A high-efficiency expression technology of Pichia pastoris, which is applied in the fields of biotechnology and genetic engineering, can solve the problems of ineffective secretion and high-copy strain production performance cannot be effectively exerted, so as to achieve the production performance, improve the level, and reduce accumulation Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
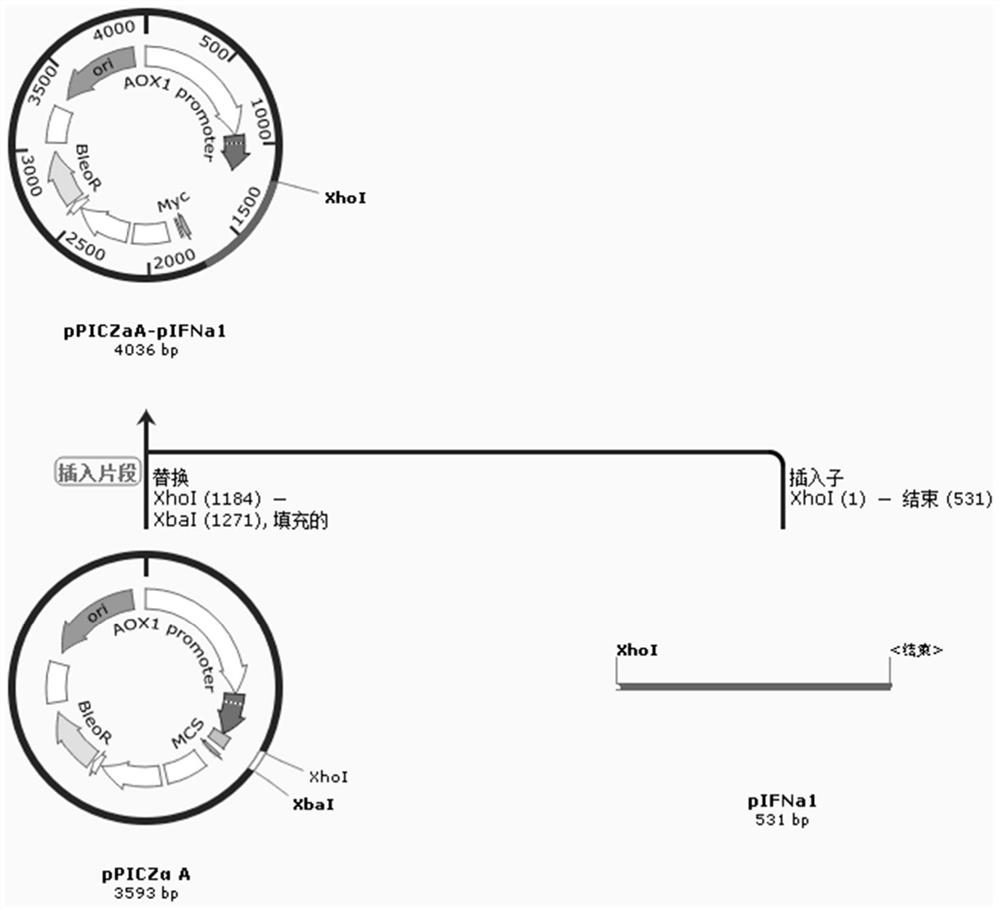
[0027] Example 1 Construction of porcine α1 interferon expression vector
[0028]1.1 Based on Pichia pastoris codon bias to optimize the pig's α1 interferon expression gene coding sequence according to Pichia pastoris codon bias (https: / / sg.idtdna.com / site / account / login?returnurl= %2FCodonOpt) designed the porcine α1 interferon gene sequence, which was synthesized by Jiangsu GenScript Biotechnology Co., Ltd., and its corresponding amino acid sequence is shown below (SEQ ID No.4).
[0029] CDLPQTHSLA HTRALRLLAQ MRRISPFSCL DHRRDFGSPH EAFGGNQVQK AQAMALVHEMLQQTFQLFST EGSAAAWNES LLHQFCTGLD QQLRDLEACV MQEAGLEGTP LLEEDSILAV RKYFHRLTLYLQEKSYSPCA WEIVRAEVMR SFSSSRNLQD RLRKKE
[0030] The optimized pIFNa1 sequence is (SEQ ID No.1):
[0031] aaaagagagg ctgaagcttg tgacttacca caaacccact ccttggctca cactagagctttgcgtctat tagctcagat gagaaggatt tcacccttta gttgtctgga tcataggcgt gacttcggatcaccacatga agccttcggt ggaaaccaag tacagaaggc ccaagcaatg gctctggtac atgagatgttgcagcaaacc ttccagttat tctccaccga...
Embodiment 2
[0040] Example 2 Construction of Pichia pastoris engineering bacteria secreting and expressing porcine α1 interferon
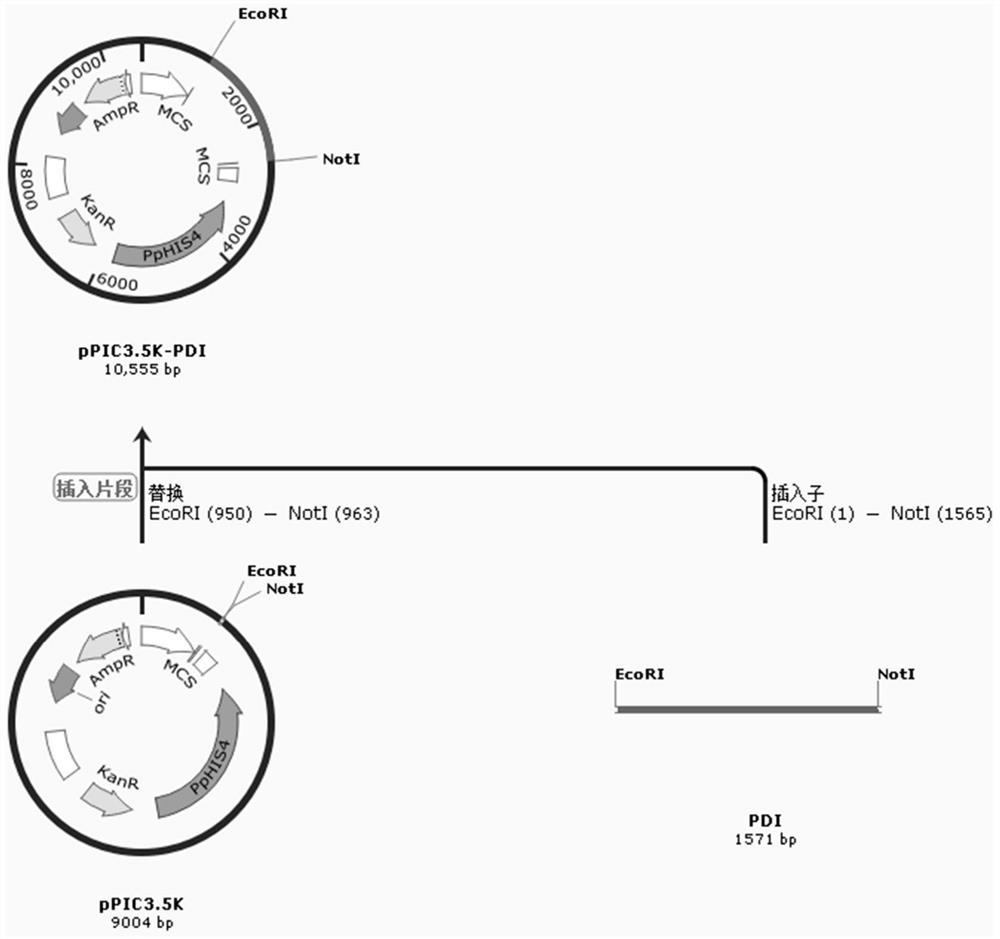
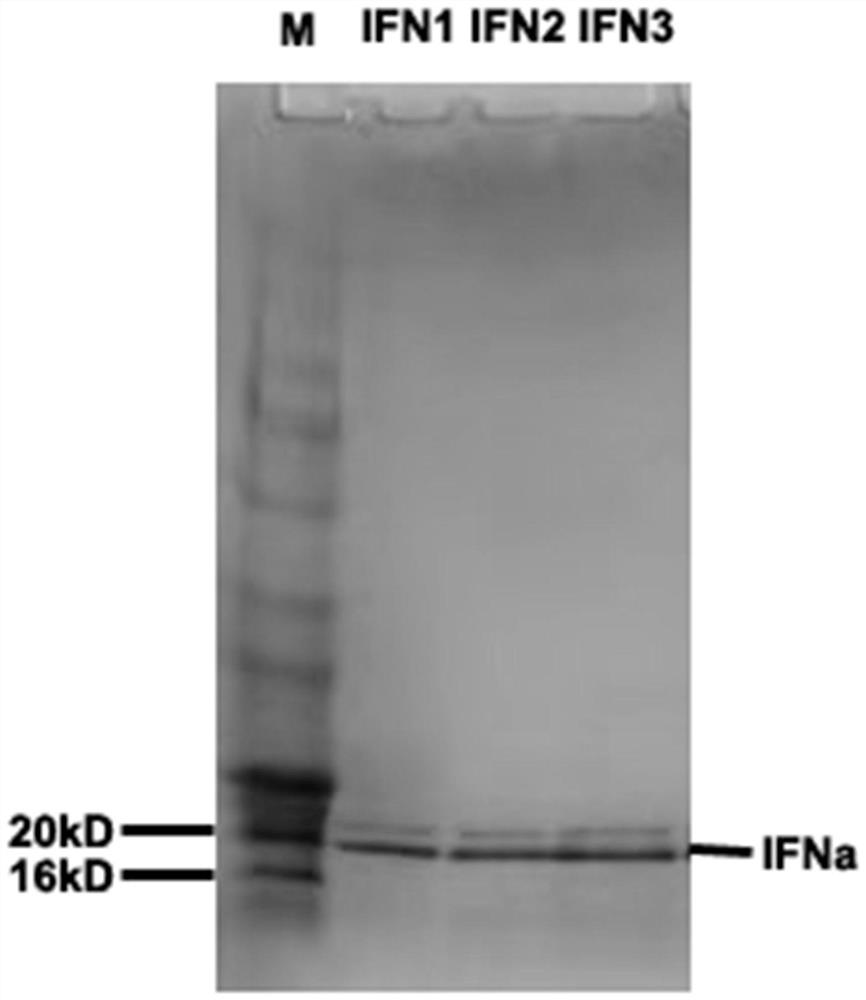
[0041]Digest the pPICZaA-pIFNa1 expression vector with SalI restriction endonuclease, cut the gel to recover the linearized expression vector, and use a voltage of 1.6kV to electrotransform into X33 Pichia competent cells or other Pichia such as GS115, KM71 and other strains In this method, the bacterial solution is spread on a YPG plate (containing 0.4 mg / ml bleomycin), and cultured at 30° C. for 2-3 days until a single colony grows. Pick a single colony, copy it to a YPG agar plate containing 0.8mg / ml bleomycin, and culture it at 30°C for 2-3 days; when the colony grows, culture it in a deep-well culture plate and induce the expression of 72 with 1.5% methanol After 1 hour, 20 μL of the supernatant of the fermentation broth was taken for SDS electrophoresis and stained to observe the interferon expression bands. As above, copy the colony grown on the YPG pl...
Embodiment 3
[0044] Example 3 Induced Expression of Recombinant Porcine Interferon α1
[0045] The Pichia strain pIFNa1-02 expressing porcine α1 interferon prepared in Example 2 was picked, inoculated in 25 mL of BMGY medium, and cultured at 30° C. and 220 rpm for 24 hours to prepare a primary seed solution. Inoculate 20mL of primary seed liquid into 200mL of BMGY medium to prepare secondary seed liquid, and culture at 30°C and 220rpm for 24 hours. All secondary seed liquids were inoculated in 5L fermenters (2L BSM medium), controlled temperature was 30°C±0.5°C, dissolved oxygen was 20%±5%, and pH=5.0±0.5. After the basal glycerol was exhausted, 10% glycerol was added continuously, until the dissolved oxygen rose to 100% after the glycerol was exhausted, starvation was performed for half an hour, methanol was added to induce the expression of Plectasin, and the total induction was 72 hours, centrifuged (6000×g, 5min ) to take the fermentation supernatant for detection.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration gradient | aaaaa | aaaaa |
| concentration gradient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com