A kind of mutant of serine hydroxymethyltransferase and use thereof
A serine hydroxymethyl and transferase technology, applied in the biological field, can solve problems such as difficulty in confirming the impact of secondary mutations and hindering the improvement of strains
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
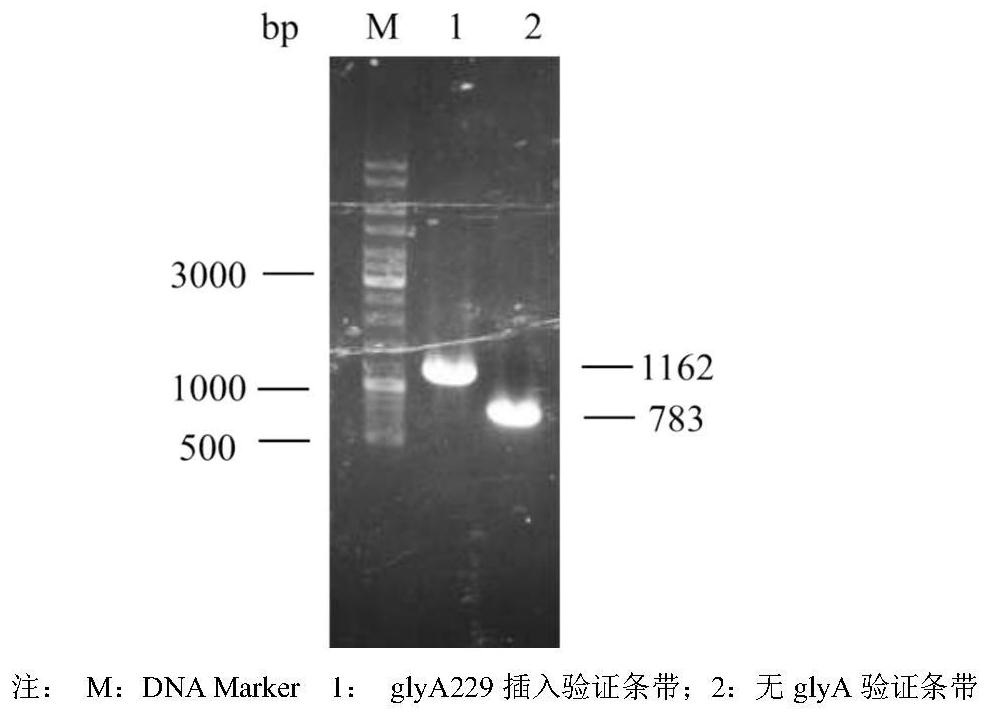
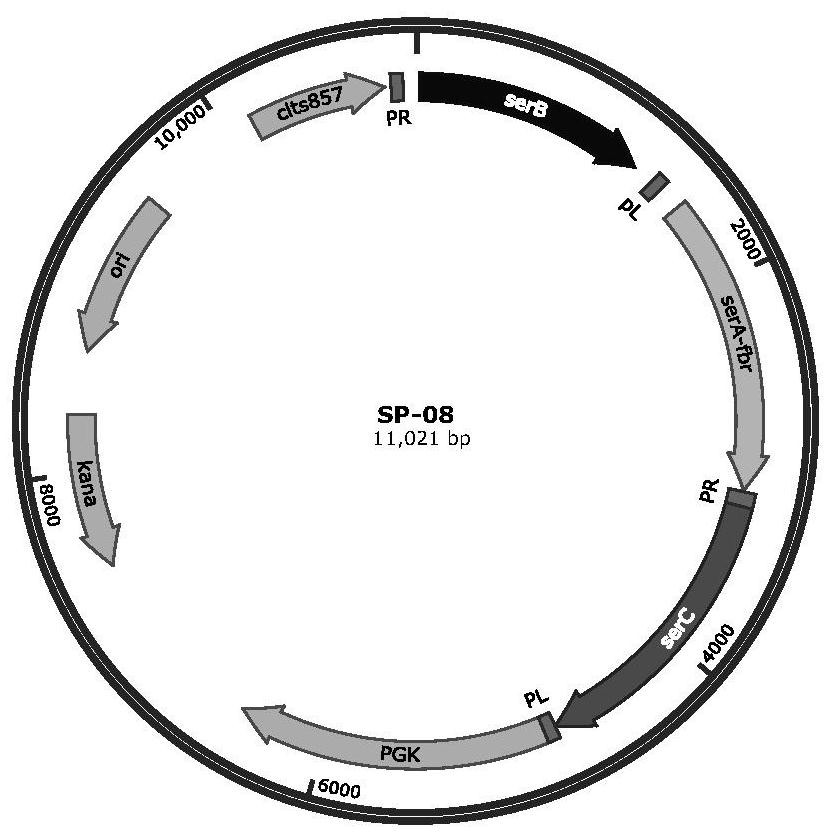
[0045] Construction of serine hydroxymethyltransferase site-directed mutant engineering bacteria
[0046] Construction of BL21(DE3) / pT 7-7-glyA: The genome of the wild-type strain E.coli W3110 (ATCC 23275) was used as the template, and the primers glyA-F / R (see Table 1 for the sequence) were used to synthesize E.coli E.coli. The serine hydroxymethyltransferase expression gene glyA in W3110 (ATCC 23275) (the glyA gene sequence is shown in the sequence 2682276-2683529 in Genbank accession number U00096.2) was amplified by PCR, verified by electrophoresis, and purified by electrophoresis gel recovery. The glyA fragment. The purified glyA fragment and pT 7-7 plasmid (Addgene, MA, USA) were double digested with NdeI and HindIII, respectively, and the double digested product of the glyA fragment and the pT 7-7 plasmid were subjected to T4 digestion. Ligase overnight at 4°C. The ligation product was transformed into Escherichia coli BL21 (DE3), spread on the LB solid plate containi...
Embodiment 2
[0059] Induced expression of serine hydroxymethyltransferase parent engineering bacteria and serine hydroxymethyltransferase site-directed mutant engineering bacteria:
[0060] The serine hydroxymethyltransferase parent engineering bacteria and the serine hydroxymethyltransferase mutant engineering bacteria in Example 1 were respectively inoculated into the LB liquid medium containing the final concentration of 100 μg / mL ampicillin, and cultured at 37°C for 8 hours, inoculated into fresh TB liquid medium containing a final concentration of 100 μg / mL ampicillin with an inoculum volume of 1% by volume, and cultured at 37°C at 200 rpm to OD=0.5 to induce induction by adding 0.1 mM IPTG, and continued. Incubate for 16h. The fermentation broth was centrifuged at 4°C and 10000rpm for 10min, the supernatant was discarded, the cells were washed with phosphate buffer and then centrifuged at 10000r / min for 10min, the supernatant was discarded, and the cells obtained after washing twice ...
Embodiment 3
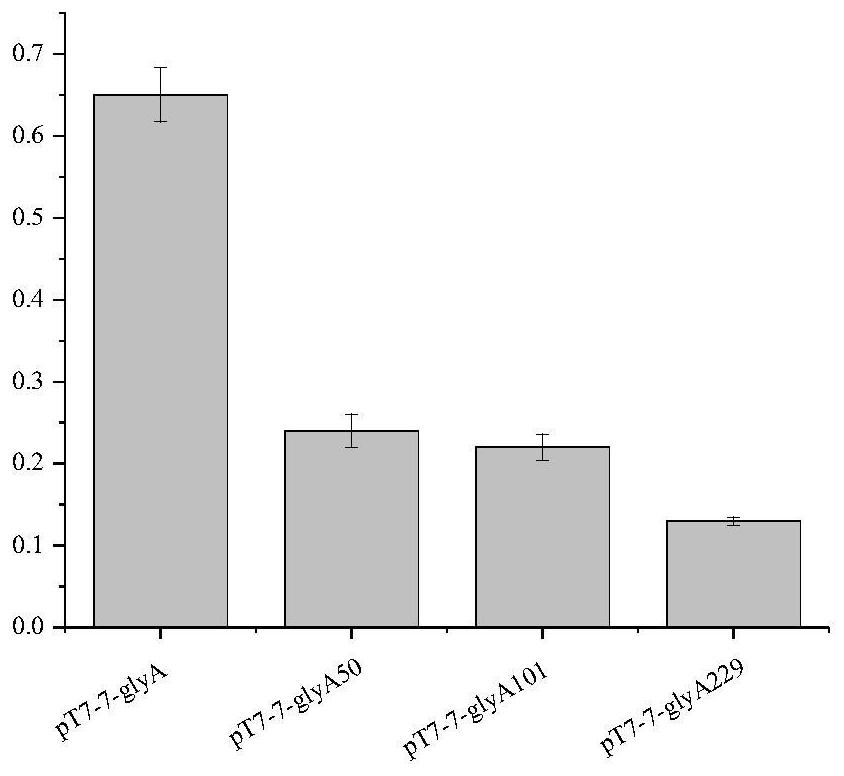
[0062] Serine hydroxymethyltransferase enzyme activity assay:
[0063] Take the serine hydroxymethyltransferase parent engineering bacteria and the serine hydroxymethyltransferase mutant engineering bacteria wet thalline prepared by the method of Example 2 as a catalyst, press 1mg wet thalli / 1mL substrate solution (50mmol / L DL- Phenylserine, 30 μmol / L pyridoxal phosphate) to prepare a reaction solution, the reaction solution was added with 0.03 g / L cetyltrimethylammonium bromide (CTAB) in a certain proportion to treat the bacteria, and the reaction solution was shaken at 30 °C for 1 h. Centrifuge at 5000r / min for 10min, take the supernatant, measure the absorbance value at A279, and determine the benzaldehyde concentration in the reaction solution according to the A279-benzaldehyde concentration regression equation. In 1 L of substrate solution at 30°C, U is 1 g of wet cells to produce 1 mol of benzaldehyde for 1 h. Using serine hydroxymethyltransferase enzyme activity unit a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com