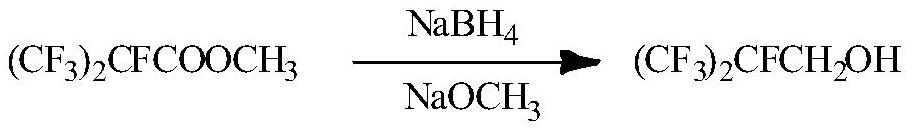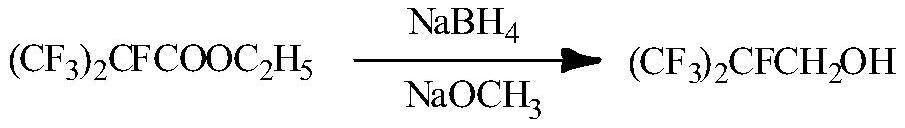Preparation method of heptafluoroisobutyl alcohol
A technology of heptafluoroisobutanol and perfluoroisobutyrate, applied in the field of preparation of heptafluoroisobutanol, can solve problems such as hindering the development and development of downstream products, unsuitable for large-scale production, harsh reaction conditions, etc. Promote and solve the effects of high cost and short synthetic route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
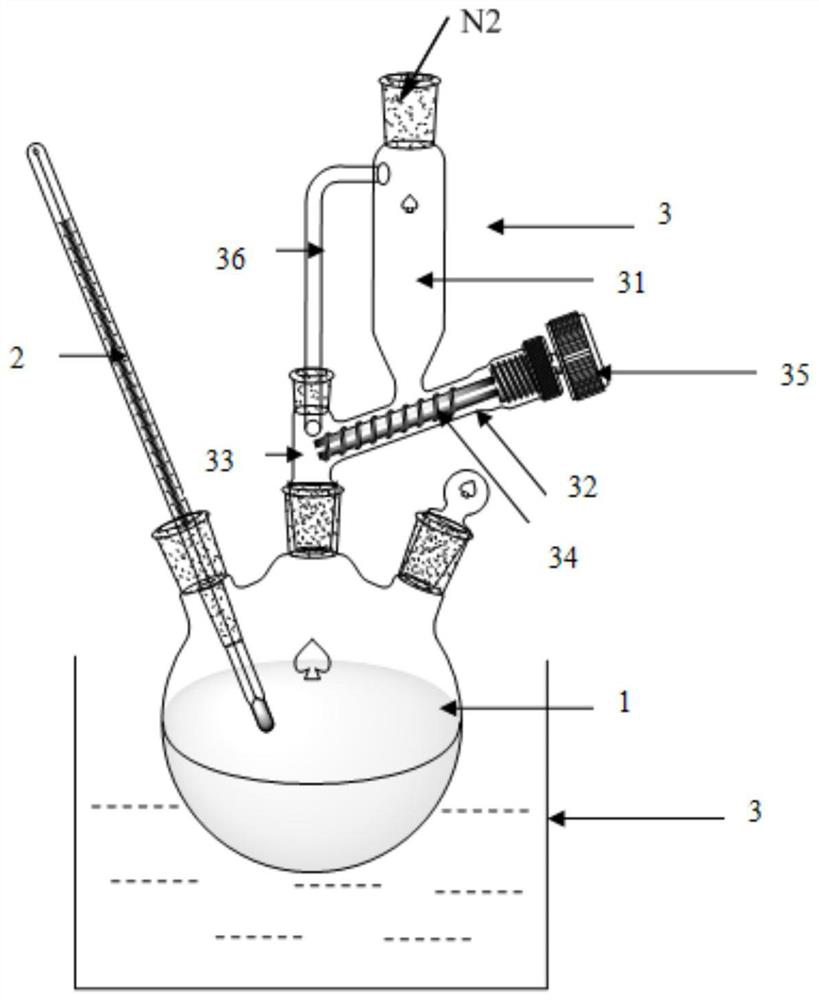
Image
Examples
Embodiment 1
[0017] Example 1. Methyl perfluoroisobutyrate (30g) was dissolved in anhydrous methanol (30mL), cooled to 0°C, sodium methoxide (7.3g) was added, and after stirring in an ice-water bath for 30 minutes, boron was slowly added in batches Sodium hydride (5 g) was added and stirred evenly, and the reaction was carried out at room temperature for 10 h, and the reaction was completed as detected by infrared spectroscopy. The solvent of the reaction solution was first distilled off, then washed with brine, dried over sodium sulfate, and concentrated to obtain the product heptafluoroisobutanol (20 g) with a yield of 76%.
Embodiment 2
[0018] Example 2. Methyl perfluoroisobutyrate (300g) was dissolved in anhydrous methanol (300mL), cooled to 0°C, sodium methoxide (73g) was added, and after stirring in an ice-water bath for 30 minutes, hydroboration was slowly added in batches Sodium (50g), stir evenly after the addition, react at room temperature for 10h, and the reaction is complete as detected by infrared spectrum. The solvent of the reaction solution was first distilled off, then washed with brine, dried over sodium sulfate, and concentrated to obtain the product heptafluoroisobutanol (220 g) with a yield of 83%.
Embodiment 3
[0019] Example 3. Dissolve ethyl perfluoroisobutyrate (60g) in anhydrous methanol (60mL), cool to 0°C, add sodium methoxide (13g), stir in an ice-water bath for 30 minutes, slowly add hydroboration in batches Sodium (4.5g), stir evenly after the addition, react at room temperature for 10h, and the reaction is complete as detected by infrared spectrum. The solvent of the reaction solution was first distilled off, then washed with brine, dried over sodium sulfate, and concentrated to obtain the product heptafluoroisobutanol (37 g) with a yield of 75%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com