High-yield ethyl chrysanthemate preparation method
A high-yield technology of ethyl chrysanthemum acid, applied in the field of chemical technology, can solve the problem of low chemical yield, and achieve the effects of reducing poisoning and excellent environmental protection performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
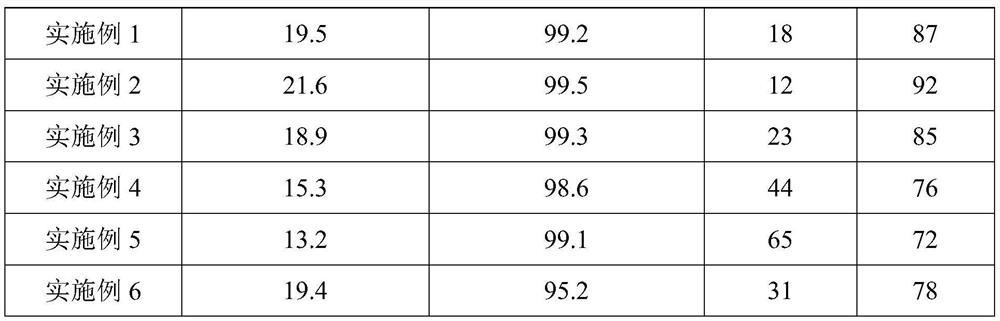
Examples
Embodiment 1
[0038] A high-yield preparation method of ethyl chrysanthemum acid, comprising the following steps:
[0039] (1) Diazotization: Put 600 parts of water, 490 parts of glycine ethyl acetate and 35 parts of glacial acetic acid into the diazotization kettle, stir to dissolve the solid, and use glacial acetic acid to keep the pH value of the diazotization reaction at 3.5~ Between 4.5, then put 800 parts of dimethyl carbonate as a solvent into the diazotization kettle, turn on the freezer, reduce the temperature of the diazotization kettle to 20-25°C, and then add the sodium nitrite aqueous solution dropwise into the diazotization kettle , the sodium nitrite addition time is controlled within 4-5 hours, after the addition, the temperature is kept for 60 minutes, after the reaction is completed, the layers are static, and the lower water layer is discharged to the waste liquid collection tank to obtain the diazonium liquid;
[0040](2) Cyclization: put 1100 parts of 2,5-dimethyl-2,4-h...
Embodiment 2
[0052] The difference between embodiment 2 and embodiment 1 is:
[0053] (1) Diazotization: Put 500 parts of water, 480 parts of glycine ethyl acetate and 36 parts of glacial acetic acid into the diazotization kettle, stir to dissolve the solid, and use glacial acetic acid to keep the pH value of the diazotization reaction at 3.5~ Between 4.5, then put 800 parts of dimethyl carbonate as a solvent into the diazotization kettle, turn on the freezer, reduce the temperature of the diazotization kettle to 20-25°C, and then add the sodium nitrite aqueous solution dropwise into the diazotization kettle , the sodium nitrite dropping time is controlled within 4-5 hours, after the dropping, keep warm for 45 minutes, after the reaction is completed, static layering, the lower water layer is discharged to the waste liquid collection tank, and the diazonium liquid is obtained;
[0054] (2) Cyclization: Put 1,100 parts of 2,5-dimethyl-2,4-hexadiene into the cyclization kettle, use 3.5 parts...
Embodiment 3
[0056] The difference between embodiment 3 and embodiment 1 is:
[0057] (1) Diazotization: Put 700 parts of water, 470 parts of glycine ethyl acetate and 37 parts of glacial acetic acid into the diazotization kettle, stir to dissolve the solid, and use glacial acetic acid to keep the pH value of the diazotization reaction at 3.5~ Between 4.5, then put 800 parts of dimethyl carbonate as a solvent into the diazotization kettle, turn on the freezer, reduce the temperature of the diazotization kettle to 20-25°C, and then add the sodium nitrite aqueous solution dropwise into the diazotization kettle , the sodium nitrite addition time is controlled within 4-5 hours, after the addition, the temperature is kept for 60 minutes, after the reaction is completed, the layers are static, and the lower water layer is discharged to the waste liquid collection tank to obtain the diazonium liquid;
[0058] (2) Cyclization: Put 1100 parts of 2,5-dimethyl-2,4-hexadiene into the cyclization kettl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com