Multi-site modifiable aggregation-induced emission quinoline nitrile derivative as well as preparation method and application thereof
A technology of aggregation-induced luminescence and derivatives, which can be used in luminescent materials, organic chemistry methods, chemical instruments and methods, etc., and can solve the problems of difficult functional modification and expansion, limited application, and easy aggregation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] (1) Synthesis of Dye I-1:
[0028]
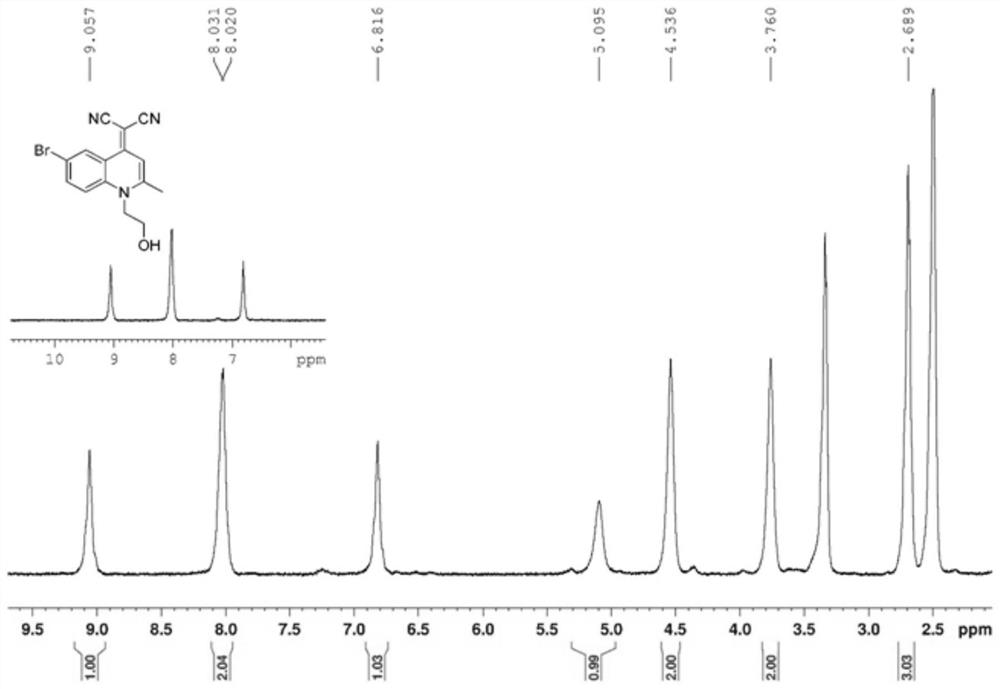
[0029] Add bromomethylquinoline (1g, 4.5mmol), iodoethanol (1.42g, 8.3mmol), ethanol (20mL) into a 50mL single-necked flask, heat at 60°C for 24h under nitrogen protection, cool to room temperature, and rotate to evaporate under reduced pressure , a black-green solid (1.6 g, 4.0 mmol) was obtained, yield: 89.9%, which was directly used in the next reaction.
[0030]
[0031] In a 50mL single-necked flask, the product of the previous step (1.6g, 4.0mmol), malononitrile (0.528g, 8.0mmol), sodium ethoxide (0.54g, 8.0mmol) and ethanol (20mL), heated at 60°C for 20h under nitrogen protection, and cooled After reaching room temperature, the solvent was removed by rotary evaporation under reduced pressure, and the product (0.4 g, 1.2 mmol) was separated by column chromatography. Yield: 30%.
[0032] 1 H-NMR (400MHz, DMSO-d 6 , ppm): δ9.0 (S, 1H, Ar-H), 8.0 (d, J=4.4Hz, 1H, Ar-H), 6.8 (S, 1H, Ar-H), 5.0 (S, 1H, OH), 4.5(S, 2H, CH ...
Embodiment 2
[0061] Absorption and Fluorescence Spectrum of Dye I-3 in Ethanol Water System
[0062] The dye I-3 prepared in Example 1 was dissolved in analytically pure dimethyl sulfoxide to make 1.0 × 10 -3 stock solution of M. Then prepare 2970 μL of mixed solvents of ethanol (EtOH) / water in different proportions. Take 30 μL of the above stock solution and add it to the prepared EtOH / water mixed solvent in different proportions, mix well and transfer to an optical quartz cuvette (10×10mm) to test its absorption and fluorescence spectra. Such as Figure 4-6 As shown, the dye I-3 exhibits a broad absorption peak at 400-550nm, and the maximum absorption wavelength is located at 450nm; with 450nm as the excitation wavelength, the maximum emission peak of the dye I-7 is approximately located at 720nm, located in the near-infrared region. The Stokes shift reaches 270nm; and the dye I-3 has no fluorescence in pure water and has the potential of biological application, and its fluorescence i...
Embodiment 3
[0064] Dye I-3 for fluorescent labeling of bovine serum albumin (BSA)
[0065] In order to study the response of dye I-3 to BSA, we first prepared 2970 μL of different concentrations of BSA (0, 40, 80, 120, 160, 200, 240, 480, 800 μg / mL) in PBS (pH=7). Then add 30 μL of the stock solution in real-time example 2 to each portion, mix well and quickly transfer to an optical cuvette to measure its absorption and fluorescence spectra. Such as Figure 7-9 As shown, as the concentration of BSA increases, the absorption spectrum gradually broadens, and the fluorescence intensity increases gradually, and the fluorescence enhancement at 720nm has a very good linear relationship (R 2 = 0.996).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com