Rift valley fever virus humanized monoclonal antibody and application thereof
一种抗体、抗原的技术,应用在医药领域,能够解决临床数据不多、成本问题、不可能大范围接种等问题,达到高应用价值、预防感染的效果
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
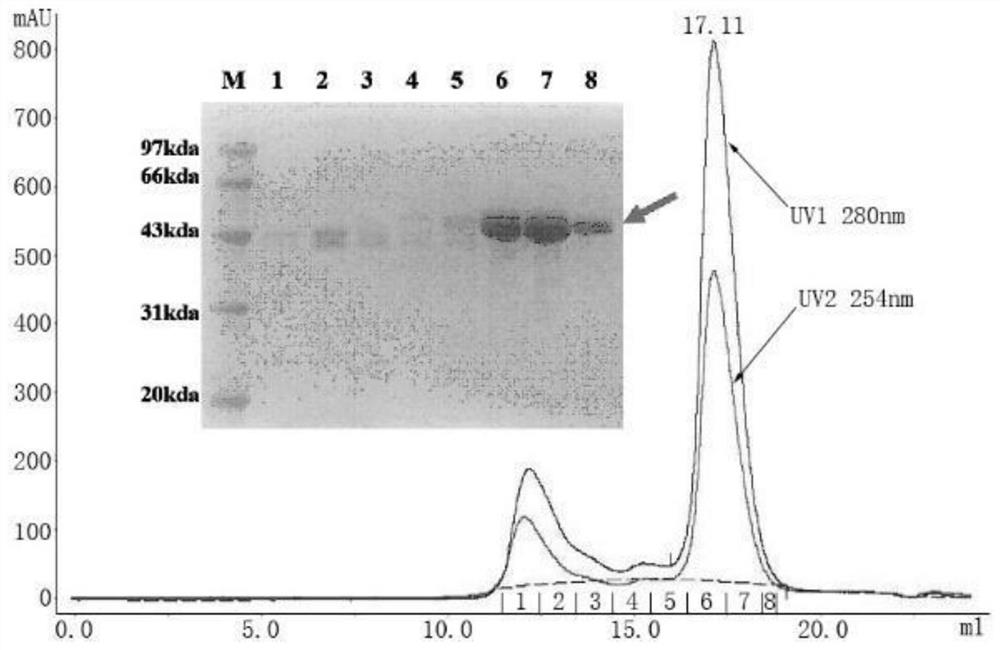
[0069] Example 1: Expression and purification of RVFV Gn and Gc proteins
[0070] The genes encoding the RVFV Gn (amino acid sequence shown in SEQ ID NO:23) and Gc (amino acid sequence shown in SEQ ID NO:24) proteins were respectively connected to the pFastBac1 vector, wherein Gn was connected to the N-terminal of the Gc protein gene There is an insect cell membrane protein Gp67 signal peptide sequence for antibody secretion; a His tag is attached to the C-terminus, which is convenient for purification and staining of B cells; all coding genes have been optimized for insect cell codons.
[0071] Transform Escherichia coli DH10Bac competent cells with the pFastBac1 vector containing the target gene, and perform blue-white screening of recombinant baculoviruses on a plate containing ampicillin, kanamycin, tetracycline and Blue-gal. After the white plaques were identified by PCR with the upstream and downstream primers of M13, the positive clones were shaken, and the recombinant ...
Embodiment 2
[0075] Example 2: Isolation of RVFV Gn and Gc protein-specific memory B cells
[0076] With the patient's informed consent, 30 mL of blood was collected and PBMCs were isolated.
[0077] Separated PBMCs in 10 7 Individual / mL density and RVFV Gn and RVFV Gc proteins with a final concentration of 100nM were incubated on ice for half an hour, then washed twice with PBS, and then incubated with the following antibodies on ice for half an hour: anti-human CD3 / PE-Cy5, anti-human CD16 / PE-Cy5, anti-human CD235a / PE-Cy5, anti-human CD19 / APC-Cy7, anti-human CD27 / Pacific Blue, anti-human CD38 / APC, anti-human IgG / FITC and anti -His / PE, then washed 2 times with PBS.
[0078] The PE-Cy5-APC-APC-Cy7+Pacific Blue+FITC+PE+ cells were collected by FACSAria III sorting, and directly collected into a 96-well plate, 1 cell / well.
Embodiment 3
[0079] Example 3: Single B cell PCR and sequence analysis
[0080] The cells obtained in Example 2 were reverse-transcribed by Superscript III reverse transcriptase (Invitrogen), and reacted at 55° C. for 60 min.
[0081] Using this reverse transcription product as a template, PCR was performed with HotStar Tap Plus enzyme (QIAgen) to amplify the antibody variable region sequence (PCRa). The reaction conditions were as follows: 95°C, 5min; 95°C, 30s, 55°C (heavy chain / κ chain) / 50°C (λ chain), 30s, 72°C, 90s, 35 cycles, 72°C, 7min.
[0082] Use this as a template for another round of PCR (PCRb), the conditions are as follows: 95°C, 5min; 95°C, 30s, 58°C (heavy chain) / 60°C (κ chain) / 64°C (λ chain), 30s, 72 ℃, 90s, 35 cycles, 72℃, 7min.
[0083] 1.2% agarose gel electrophoresis to separate the PCR products. The bands with a size of 400-500 bp were recovered and sent to the sequencing company for sequencing. The sequencing results were analyzed with IMGT online software.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com