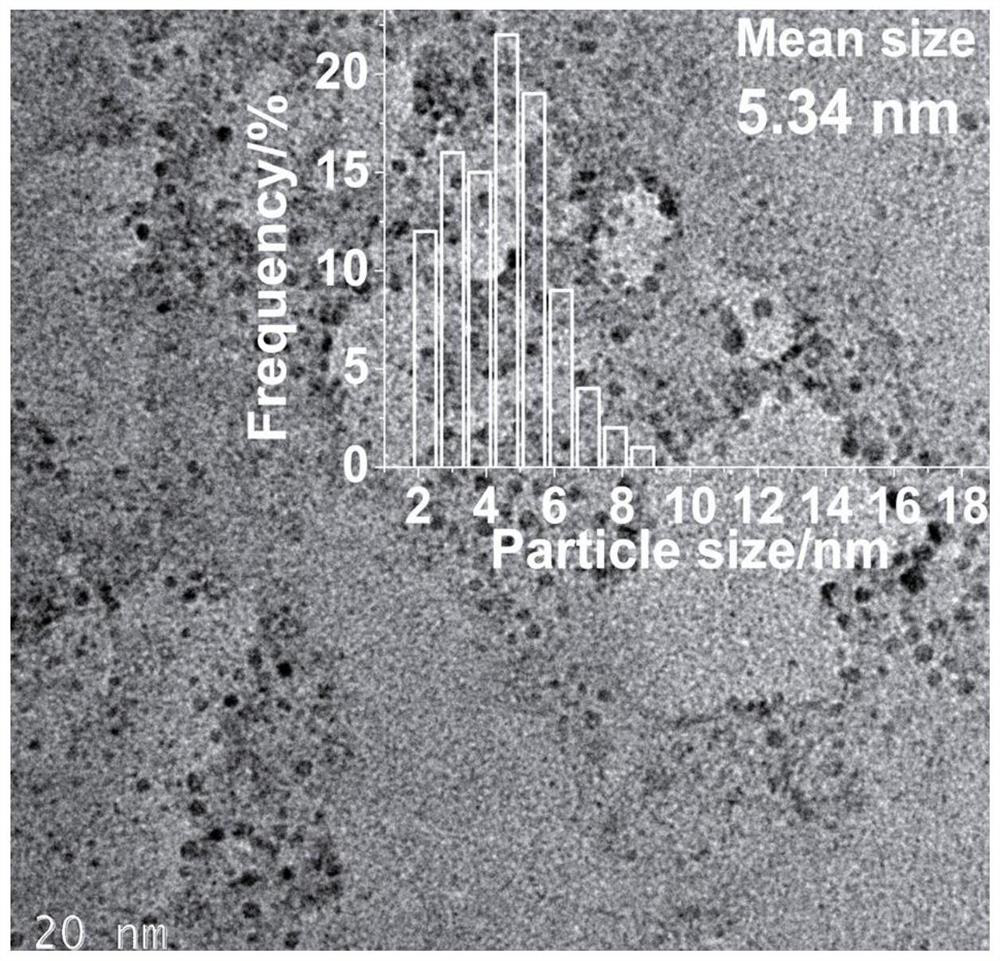
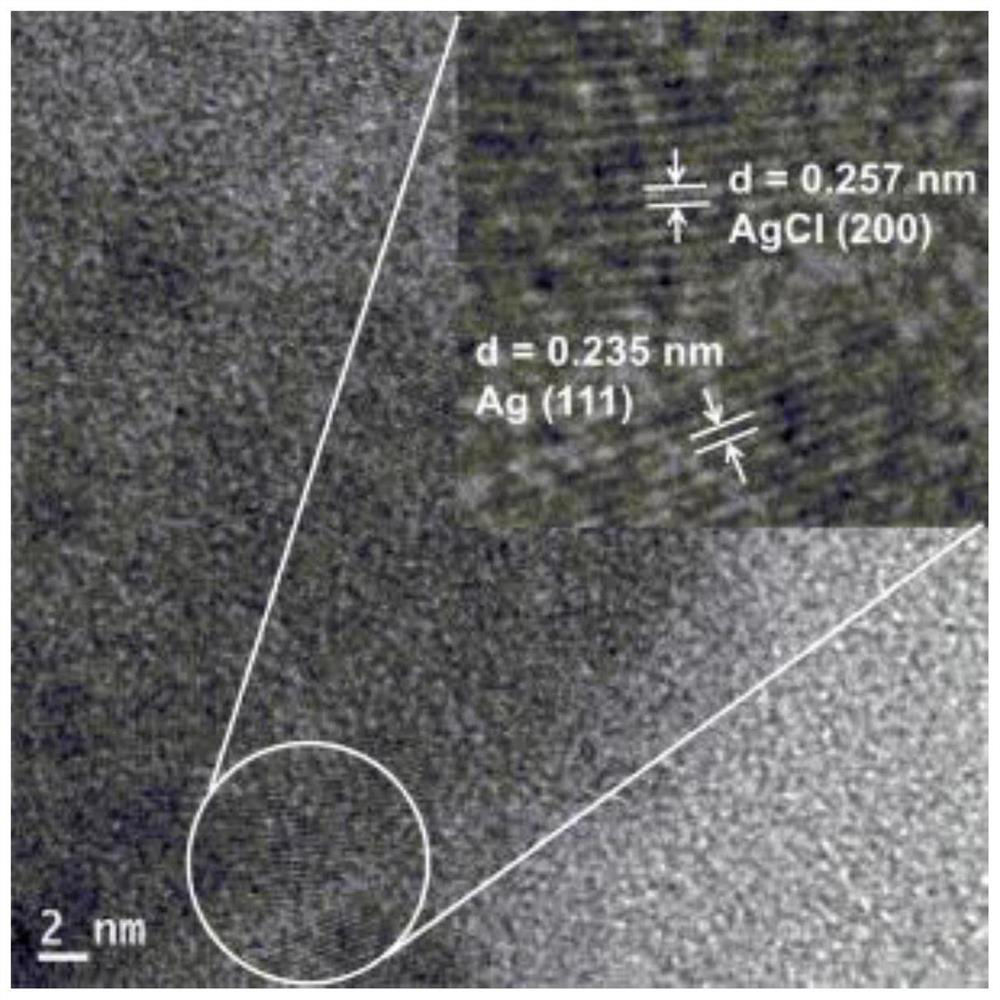
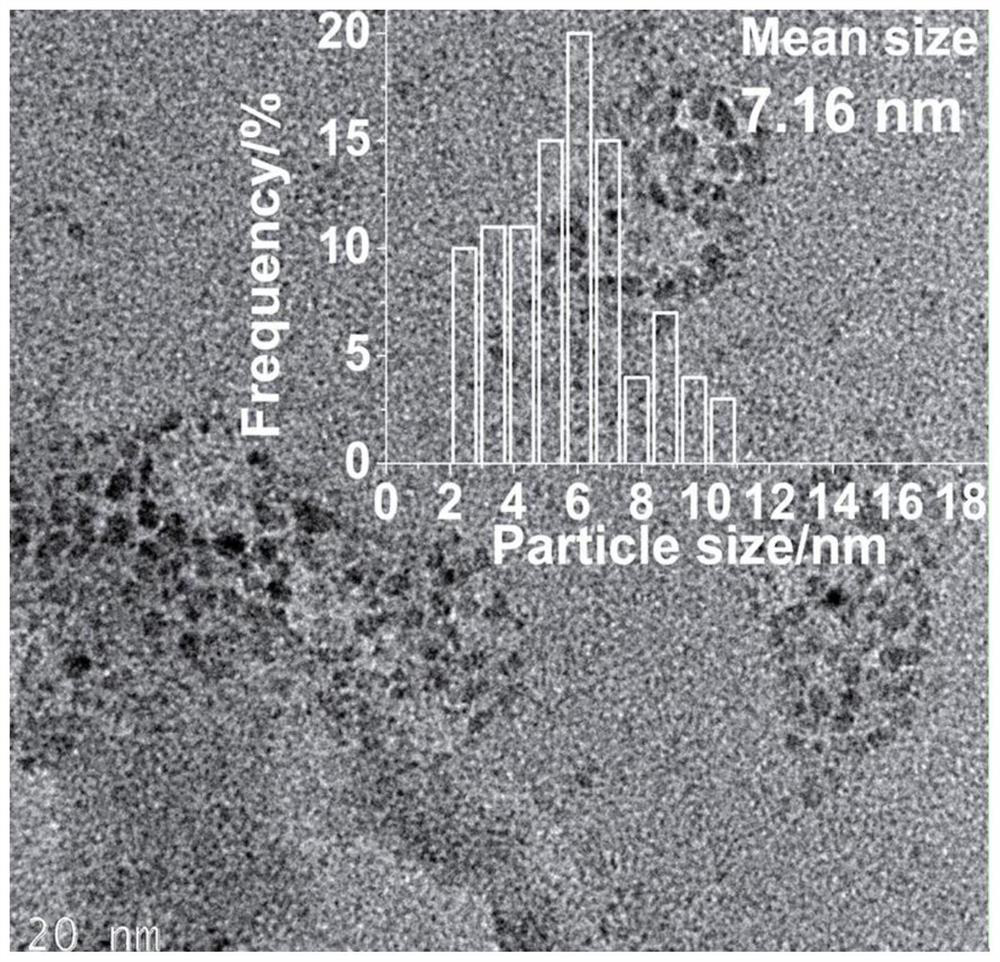
Preparation method and application of cellulose hydrogel-based nano-silver/silver chloride
A hydrogel-based, cellulose technology is applied in the field of preparation of cellulose hydrogel-based nano-silver/silver chloride, which can solve the problems of large particle size of nano-silver particles, and achieve mild conditions, low preparation cost, and equipment. request simple effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The green preparation of cellulose hydrogel-based nano-silver / silver chloride of the present invention comprises the following steps:
[0056] (1) Preparation of cellulose solution
[0057] i. DMAC heat activation: add DMAC to an appropriate amount of cotton, and heat activate it at 160 °C for 40 minutes to obtain activated cotton.
[0058] ii. Pressing with a hydraulic press: press the activated cotton at 200°C for 1 min to obtain absorbent cotton.
[0059] iii. Stirring at high temperature: at 100 ℃, put 1g of absorbent cotton in 99g of 8.5 wt% LiCl / DMAC polar solution, stir for 3 hours, cool down to room temperature, continue to stir until dissolved, and set aside to obtain a clear cellulose solution.
[0060] (2) Add 50 g of cellulose solution and 0.6 g of tannic acid in 30 mL of DMAC to the three-necked flask, and add the DMAC solution of tannic acid dropwise into the three-necked flask under stirring at 25 °C, and the mixture Stir at 25 °C for 2 h to obtain reac...
Embodiment 2
[0069] The cellulose hydrogel-based nano silver / silver chloride prepared in Example 1 was used to reduce aromatic nitro compounds.
[0070] The cellulose hydrogel-based nano-silver / silver chloride prepared in Example 1 was dispersed in deionized water to prepare a 2 mg / mL catalyst suspension. Add 0.5 mL of the prepared catalyst to 3 mL of 0.2 mM p-nitrophenol, p-nitroaniline, and o-nitrophenol nitrate respectively, pass nitrogen gas for 20 minutes, then transfer the reaction solution to a cuvette, add 0.3 mL, 0.05 MNbH 4 , react at room temperature. The results of ultraviolet tracking reaction are as follows: Figure 11 , Figure 12 and Figure 13 . exist Figure 11 The catalytic reaction process was monitored by recording the characteristic absorption band of 4-NP at 400 nm as a function of reaction time. The intensity of the absorption peak at 400nm gradually decreases with time, and after about 5 minutes (without any stirring), the absorption peak completely disappea...
Embodiment 3
[0074] The cellulose hydrogel-based nano silver / silver chloride prepared in Example 1 was used to degrade organic dyes.
[0075] The cellulose hydrogel-based nano-silver / silver chloride prepared in Example 1 was dispersed in deionized water to prepare a 2 mg / mL catalyst suspension. Add 0.5 mL (2 mg / mL) of the prepared catalyst to 3 mL of 0.2 mM methyl orange, Congo red, alizarin yellow, and rhodamine-B respectively, pass nitrogen gas for 20 minutes, and then transfer the reaction solution to a cuvette , add 0.3 mL, 0.02M, 0.1 M, 0.2M NaBH 4 , react at room temperature. Ultraviolet tracking of the reaction results. The results of ultraviolet tracking reaction are as follows: Figure 16 , Figure 17 , Figure 18 and Figure 19 . It can be seen from the figure that methyl orange, Congo red, alizarin yellow and rhodamine-B were all reduced after about 4 min, 7 min, 13 min and 3 min (without any stirring).
[0076] Since sewage generally contains a variety of organic dyes, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com