Methylene-bridged nitrogen-rich heterocyclic compound and derivatives thereof, and preparation methods thereof
A technology of heterocyclic compounds and methylene bridges, applied in the field of preparation, methylene bridged nitrogen-rich heterocyclic compounds and their derivatives, can solve the problems of high energy and low, difficult to achieve, and mechanical sensitivity cannot be satisfied at the same time, To achieve the effect of good stability and high energy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] A method for preparing a methylene bridged nitrogen-rich heterocyclic compound of the present invention comprises the following steps:
[0034] Step 1: Dissolve 2mmol of 1-acetonitrile 5-aminotetrazole in 10ml of acetonitrile, add 2mmol of hydroxylamine solution dropwise at -5~5°C, transfer to room temperature for reaction for 10~14h after the dropwise addition, and the reaction ends Afterwards, the precipitate was filtered off to obtain compound 1, namely methylene-bridged 5-aminotetrazole and 5-amino-1,2,4-oxadiazole.
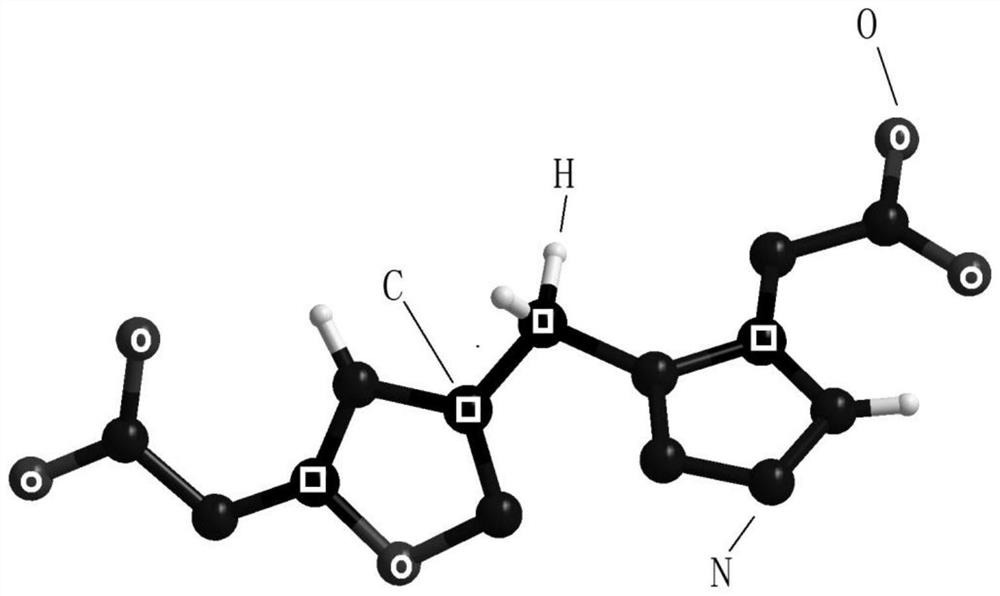
[0035] The structural formula of compound 1 is:
[0036]
[0037] Step 2: Dissolve 2 mmol of compound 1 in 10 ml of ethanol, add 2 mmol of sodium bicarbonate and stir for 5 min to form a suspension, and add 2.2 mmol of cyanogen bromide to the suspension. Stir at room temperature for 10-14h. The obtained solid was filtered, rinsed with deionized water, and dried in air to obtain a white solid, which was designated as compound 2.
[0038] The struc...
Embodiment 1
[0052] Step 1: Dissolve 2mmol of 1-acetonitrile 5-aminotetrazole in 10ml of acetonitrile, add 2mmol of hydroxylamine solution dropwise at 0°C, transfer to room temperature to react for 12h after the dropwise addition, and filter out the precipitate after the reaction. Compound 1 was obtained.
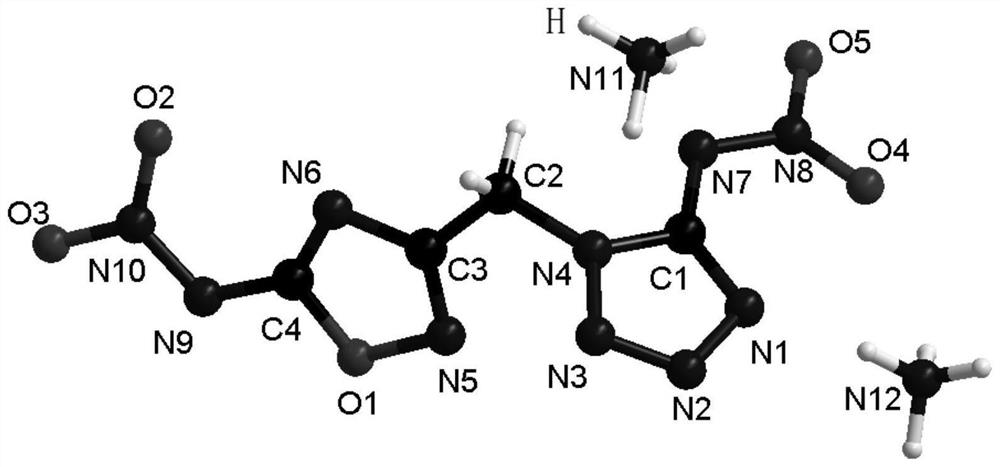
[0053] Step 2: Dissolve 2 mmol of compound 1 in 10 ml of ethanol, add 2 mmol of solid sodium bicarbonate and stir for 5 min to form a suspension, and add 2.2 mmol of cyanogen bromide to the suspension. Stir at room temperature for 12h. The resulting solid was filtered, rinsed with deionized water, and dried in air to obtain a white solid compound 2 with a yield of 90%, constant volume heat of combustion Qv=8000J / g, IS=40J, FS=360N, indicating high energy and stability it is good.
[0054] Step 3: Take 1ml of fuming nitric acid and stir at 0°C for 10 minutes, take 0.142g of compound 1 and dissolve in fuming nitric acid at 0°C, slowly return to room temperature, and oxidize for 8 hours ...
Embodiment 2
[0062] Step 1: Dissolve 2mmol of 1-acetonitrile 5-aminotetrazole in 10ml of acetonitrile, add 2mmol of hydroxylamine solution dropwise at -5°C, transfer to room temperature to react for 14h after the dropwise addition, and filter out the precipitate after the reaction , to obtain compound 1.
[0063] Step 2: Dissolve 2 mmol of compound 1 in 10 ml of ethanol, add 2 mmol of solid sodium bicarbonate and stir for 5 min to form a suspension, and add 2.2 mmol of cyanogen bromide to the suspension. Stir at room temperature for 10 h. The resulting solid was filtered, rinsed with deionized water, and dried in air to obtain compound 2 as a white solid.
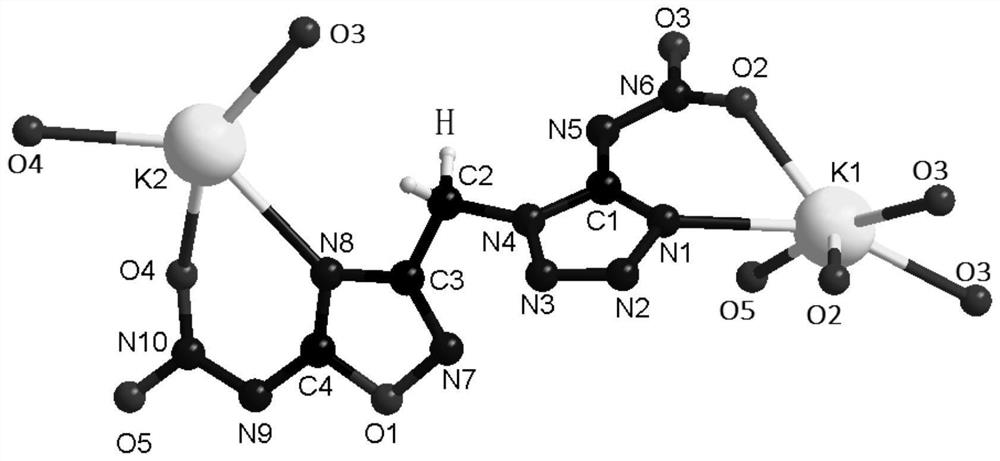
[0064] Step 3: Take 1ml of fuming nitric acid and stir at -5°C for 10min, take 0.142g of compound 1 and dissolve in fuming nitric acid at -5°C, slowly return to room temperature, and oxidize for 9h while stirring. After the reaction was completed, the reaction solution was dropped drop by drop on ice, and a white product was precipita...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com