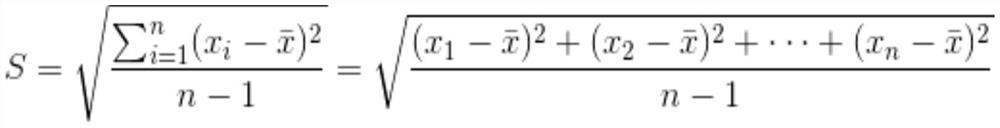Mecobalamin tablet and preparation method thereof
A technology of methylcobalamin tablets and methylcobalamin, which is applied in the fields of pill delivery, pharmaceutical formulations, medical preparations of non-active ingredients, etc., and can solve the cumbersome preparation method, large difference in particle size distribution, and increase the risk of degradation of methylcobalamin To achieve the effect of ensuring mixing uniformity, improving content uniformity, and reducing stability risks
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
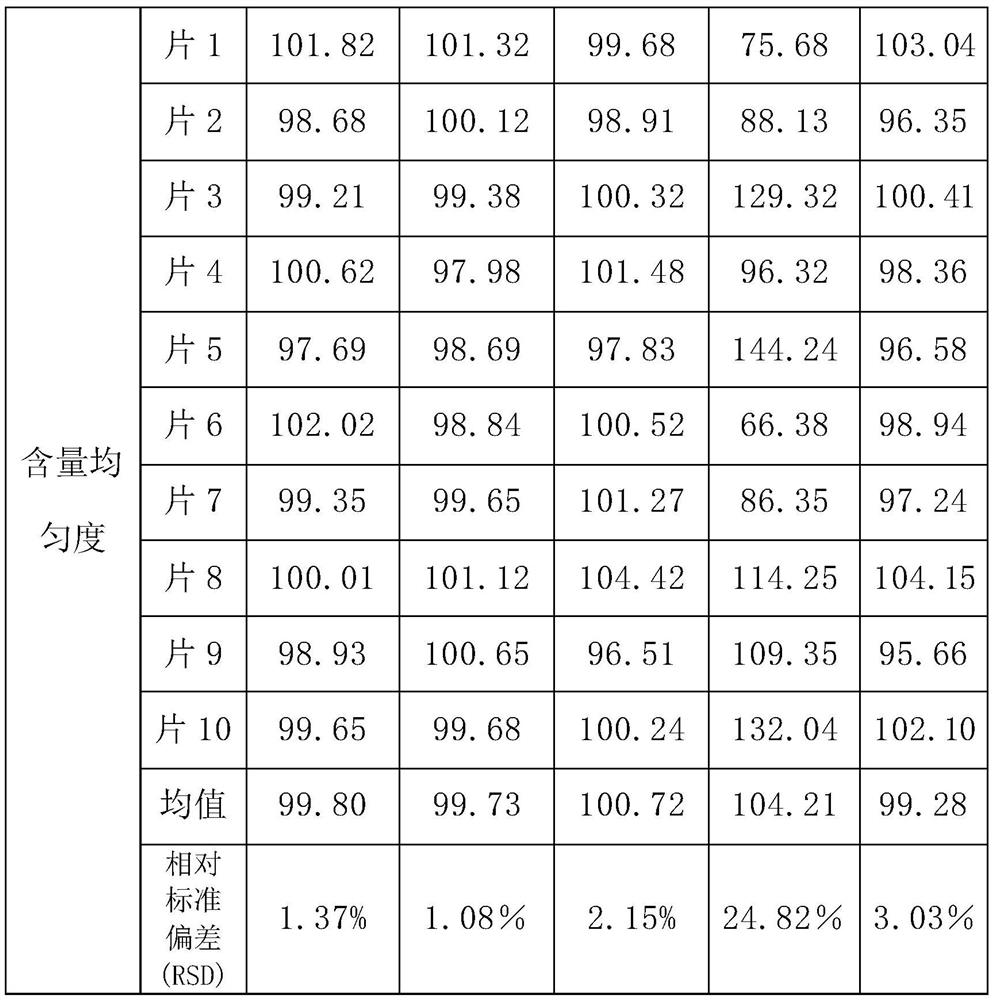
[0038] A methylcobalamin tablet, comprising 100.0 mg of a plain tablet and 5.0 mg of a film coating layer; the mass ratio of the plain tablet to the film coating layer is 100:5;
[0039]The plain tablet includes the following components by weight: 0.5 mg methylcobalamin, 60.0 mg direct-pressed lactose, 10230.0 mg microcrystalline fiber, 8.5 mg pregelatinized starch, and 1.0 mg calcium stearate (lubricant);
[0040] The film coating layer comprises the following components by weight: 1.5 mg of hydroxypropyl methylcellulose, 2.0 mg of polyethylene glycol, 1.0 mg of titanium dioxide, 0.25 mg of yellow iron oxide, and 0.25 mg of red iron oxide.
[0041] The preparation method of above-mentioned methylcobalamin tablet, comprises the steps:
[0042] One, the preparation of plain tablet:
[0043] (1) Accurately weigh 0.5 mg of methylcobalamin, 60.0 mg of direct-pressed lactose, 10230.0 mg of microcrystalline fiber, 8.5 mg of pregelatinized starch, and 1.0 mg of calcium stearate acco...
Embodiment 2
[0056] A methylcobalamin tablet, comprising 100.0 mg plain tablet and 7.0 mg film coating layer; the mass ratio of the plain tablet to the film coating layer is 100:7;
[0057] The plain tablet comprises the following components by weight: methylcobalamin 0.5mg, direct compression lactose 42.5mg, microcrystalline fiber 10245.0mg, cornstarch 5.0mg, pregelatinized starch 5.0mg, calcium stearate (lubricant) 1.0 mg, stearic acid 1.0mg;
[0058] The film coating layer comprises the following components by weight: 2.45 mg of hydroxypropyl methylcellulose, 2.24 mg of polyethylene glycol, 1.19 mg of titanium dioxide, 0.56 mg of yellow iron oxide, and 0.56 mg of red iron oxide.
[0059] The preparation method of above-mentioned methylcobalamin tablet, comprises the steps:
[0060] One, the preparation of plain tablet:
[0061] (1) Accurately weigh 0.5 mg of methylcobalamin, 42.5 mg of direct-pressed lactose, 10245.0 mg of microcrystalline fiber, 5.0 mg of cornstarch, 5.0 mg of pregel...
Embodiment 3
[0074] A methylcobalamin tablet, comprising 100.0 mg of a plain tablet and 10.0 mg of a film coating layer; the mass ratio of the plain tablet to the film coating layer is 100:10;
[0075] The plain tablet includes the following components by weight: 0.5 mg of methylcobalamin, 45.0 mg of direct-pressed lactose, 10245.0 mg of microcrystalline fiber, 7.5 mg of pregelatinized starch, and 2.0 mg of stearic acid;
[0076] The film coating layer comprises the following components by weight: 2.0 mg of hydroxypropyl methylcellulose, 2.5 mg of polyethylene glycol, 3.0 mg of titanium dioxide, 1.0 mg of yellow iron oxide, and 1.5 mg of red iron oxide.
[0077] The preparation method of above-mentioned methylcobalamin tablet, comprises the steps:
[0078] One, the preparation of plain tablet:
[0079] (1) Accurately weigh 0.5 mg of methylcobalamin, 45.0 mg of direct-pressed lactose, 10245.0 mg of microcrystalline fiber, 7.5 mg of pregelatinized starch, and 2.0 mg of stearic acid accordin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com