Application of 4-amino-pyrrolotriazine derivative in preparation of anti-pulmonary fibrosis preparation
A triazine derivative, pulmonary fibrosis technology, applied in the directions of organic chemistry, drug combination, heterocyclic compound active ingredients, etc. It can solve the problems of poor long-term efficacy and other problems, and achieve the effects of excellent biological activity, short reaction time and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
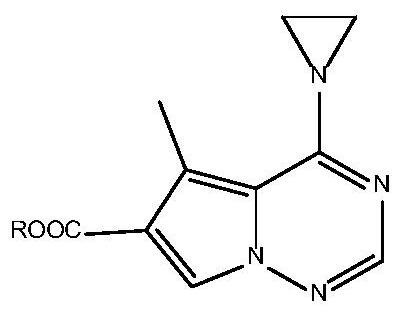
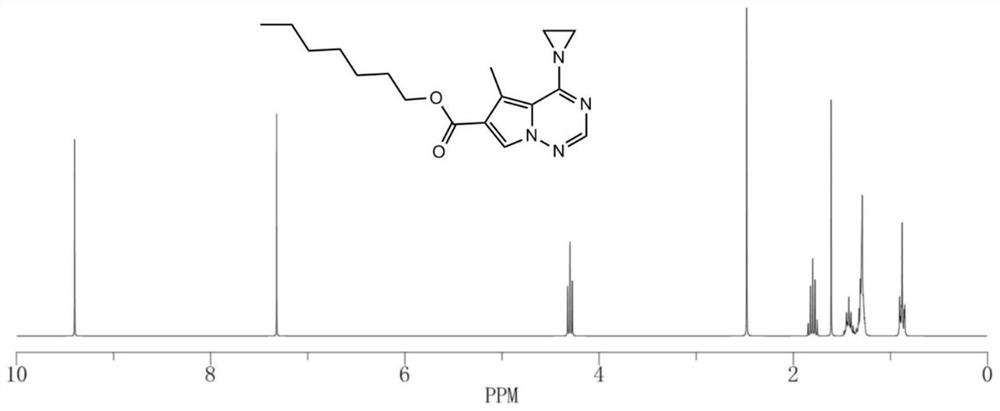
[0068] Example 1: Heptyl 5-methyl-4-(1-aziridine)pyrrolo[2,1-f][1,2,4]triazine-6-carboxylate:
[0069] This embodiment provides a kind of heptyl 5-methyl-4-(1-aziridine)pyrrolo[2,1-f][1,2,4]triazine-6-carboxylate, its synthesis steps as follows:
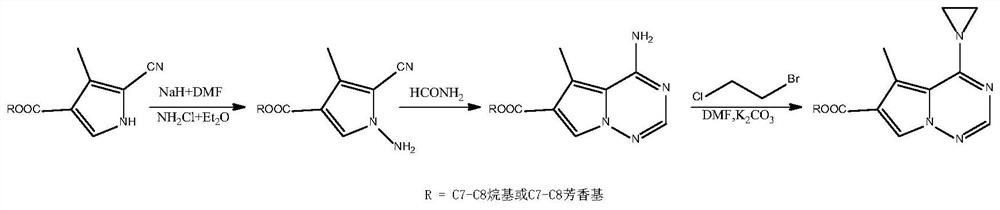
[0070] 1) Dissolve 1g of 3-methyl-2-cyanopyrrole-4-heptyl carboxylate completely in 6mL of DMF, cool to -25°C, add 0.6gNaH under stirring, and keep stirring at 300r / min for 45min; at 10mL / min Slowly add 150mL of 0.15mol / LNH 2 Cl diethyl ether solution, keep stirring at constant temperature for 2h, raise the temperature to room temperature at 5°C / min and stir for 1h; saturated Na 2 S 2 o 3 After quenching the reaction with an aqueous solution, the organic phase was obtained, the aqueous phase was extracted 3 times with ether, all the organic phases were combined, washed with saturated brine, dried and concentrated over anhydrous sodium sulfate, and separated by silica gel column chromatography (the mobile phase was ethyl acetate: ...
Embodiment 2
[0074]Example 2: Octyl 5-methyl-4-(1-aziridine)pyrrolo[2,1-f][1,2,4]triazine-6-carboxylate:
[0075] This embodiment provides a kind of octyl 5-methyl-4-(1-aziridine)pyrrolo[2,1-f][1,2,4]triazine-6-carboxylate, its synthesis steps With embodiment 1, specifically as follows:
[0076] 1) Dissolve 1.5g of 3-methyl-2-cyanopyrrole-4-octyl carboxylate completely in 9mL of DMF, cool to -22°C, add 0.9gNaH under stirring, and keep stirring at 450r / min for 50min; Slowly add 150mL of 0.16mol / LNH 2 Cl diethyl ether solution, keep stirring at constant temperature for 2h, warm up to room temperature at 4°C / min and stir for 1h; saturated Na 2 S 2 o 3 After quenching the reaction with an aqueous solution, the organic phase was obtained, the aqueous phase was extracted 3 times with ether, all the organic phases were combined, washed with saturated brine, dried and concentrated over anhydrous sodium sulfate, and separated by silica gel column chromatography (the mobile phase was ethyl aceta...
Embodiment 3
[0080] Example 3: Benzyl 5-methyl-4-(1-aziridine)pyrrolo[2,1-f][1,2,4]triazine-6-carboxylate:
[0081] This example provides a kind of benzyl 5-methyl-4-(1-aziridine)pyrrolo[2,1-f][1,2,4]triazine-6-carboxylate, which is synthesized Step is with embodiment 1, specifically as follows:
[0082] 1) Dissolve 2g of 3-methyl-2-cyanopyrrole-4-benzoic acid benzyl completely in 10mL of DMF, cool to -24°C, add 1.2gNaH under stirring, and keep stirring at 360r / min for 60min; Slowly add 150mL of 0.18mol / LNH 2 Cl diethyl ether solution, keep stirring at constant temperature for 2h, warm up to room temperature at 3°C / min and stir for 1h; saturated Na 2 S 2 o 3 After quenching the reaction with an aqueous solution, the organic phase was obtained, the aqueous phase was extracted 3 times with ether, all the organic phases were combined, washed with saturated brine, dried and concentrated over anhydrous sodium sulfate, and separated by silica gel column chromatography (the mobile phase was e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com