Protein drug combined with immunosuppressive molecule PD-L1
An immunosuppressive molecule, the technology of PD-L1, applied in the field of medical science, can solve the problems that the development of covalent protein drugs has not made effective progress, and achieve the effect of improving binding ability and inhibiting cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
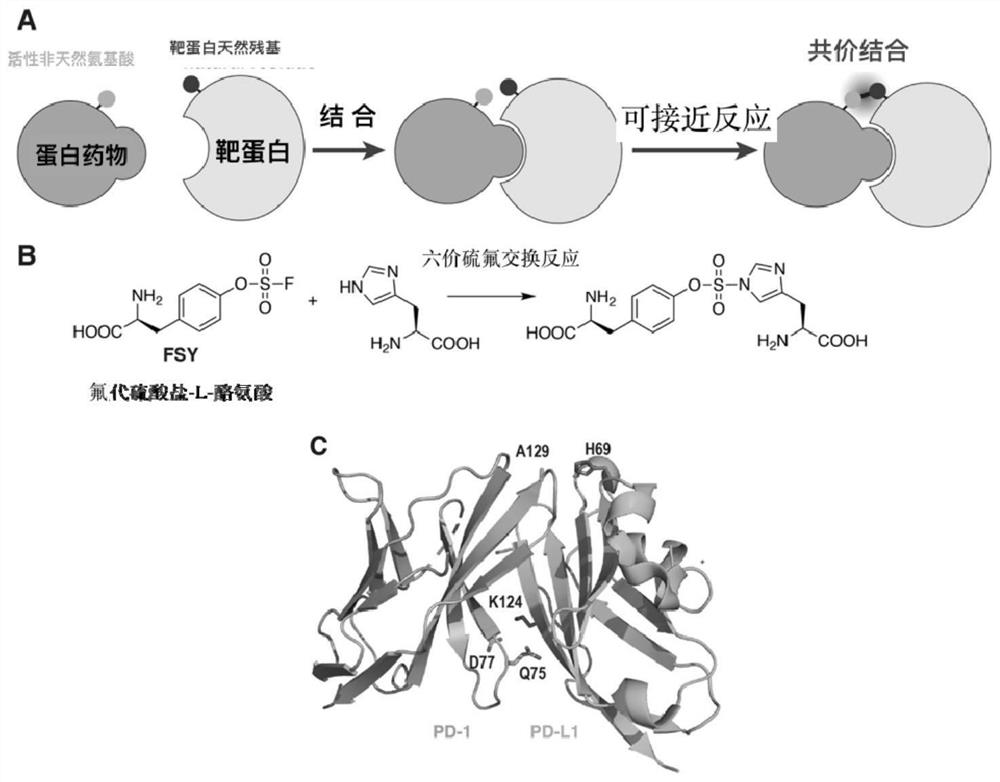
[0026] Example 1 Covalent binding of PD1FSY to PD-L1 protein
[0027]Preparation of PD1 (WT), PD1 (Q75FSY), PD1 (D77FSY), PD1 (A129FSY) protein, designed to express PD1 IgV domain (residues 32-160, cysteine 93 replaced by serine to improve protein stability sex), the structure is PD1 (WT); the TAG codons introduced at the corresponding positions of amino acids 77, 129 and 75 are PD1 (D77FSY), PD1 (A129FSY), PD1 (Q75FSY), and the optimized The DNA sequence of the His tag was cloned into the pET-26b plasmid. For PDL1 (WT), the DNA sequence from 19 to 134 residues of PDL1 protein was cloned into the pET-26b plasmid, and in PDL1 (H69A), histidine 69 was replaced with alanine, and the optimized sequence was also cloned into pET- 26b plasmid. The above plasmids were introduced into Escherichia coli BL21(DE3) by electroporation. For PD1(WT), PD-L1(WT) and PD-L1(H69A), the plasmid-transfected bacteria used 2×YT medium (add 50ug / ml kanamycin, 1mM IPTG) cultured at 37°C, for expre...
Embodiment 2
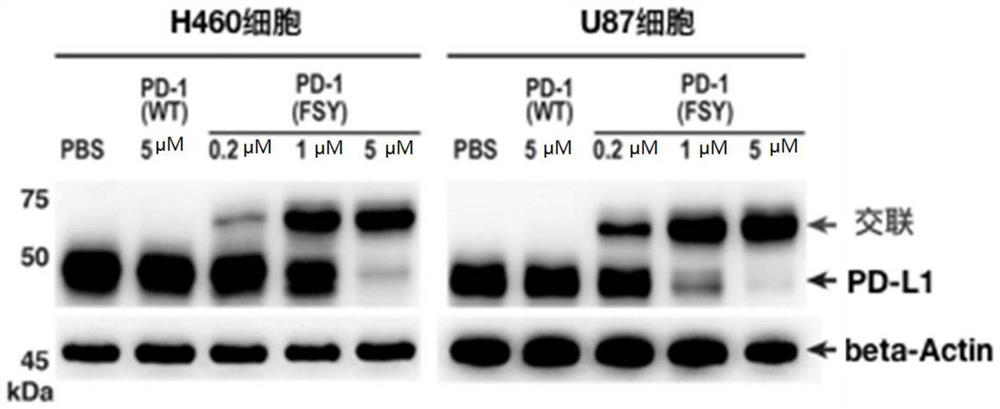
[0029] Example 2 Covalent binding of PD1FSY to tumor cell PD-L1 molecules
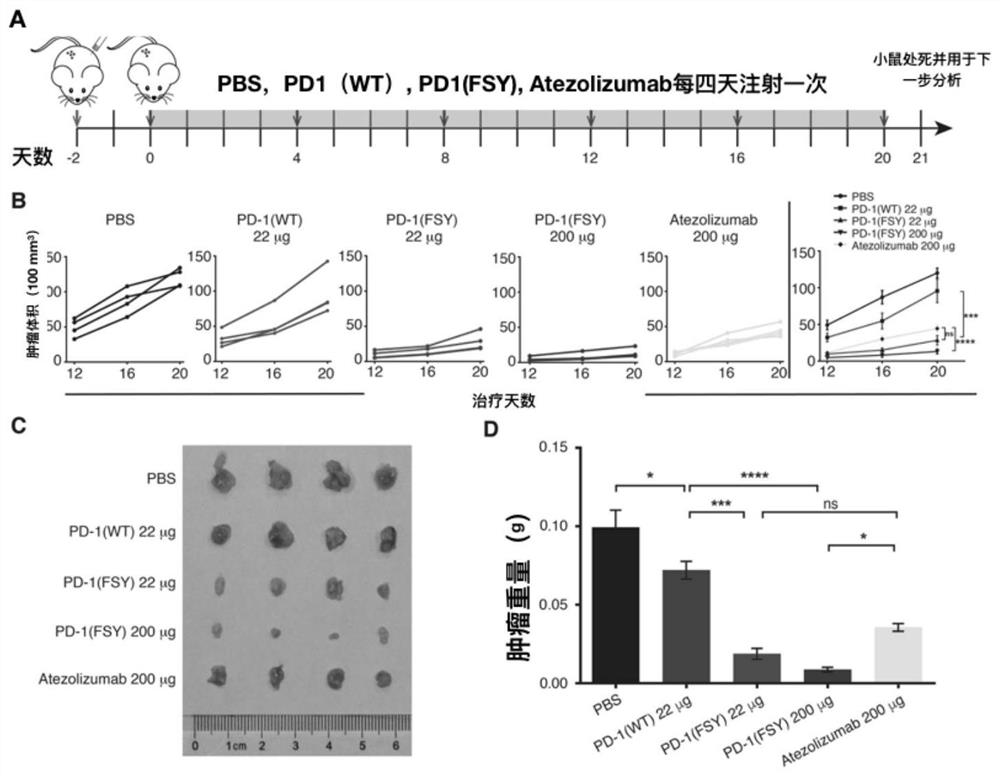
[0030] will be 5×10 5 H460 and U87 tumor cells were respectively plated in 6-well plates. After 6 hours, PD1(WT) and PD1(A129FSY) proteins were incubated with the above two kinds of cells at 37°C for 12 hours. After reaching the time point, they were digested with trypsin and collected. Cells, and use 100ul RIPA lysate to lyse the cells, extract the total protein, add 5× protein loading buffer, denature at high temperature (100°C) for 10 minutes, separate the sample by 15% SDS-PAGE gel, and transfer the protein to the NC membrane On (90V, 1.5 hours), use 5% skimmed milk to block for one hour, use anti-PD-L1 and β-Actin antibodies to incubate overnight at 4°C, wash the membrane three times with TBST and use anti-mouse or rabbit IgG with HRP The secondary antibody was incubated for 1 hour, the membrane was washed three times, and the bands were exposed. see results image 3 .
Embodiment 3
[0031] Example 3 Covalent binding of PD1FSY to tumor tissue PD-L1 molecules
[0032] Take 2×10 6 Tumor cells H460 and U87 were transplanted into severe immunodeficiency mice (NSG) by subcutaneous injection, and 10 days later, the drugs PD1 (WT) and PD1 (A129FSY) were subcutaneously injected into the mice through the tail vein and the tumor paratumours, respectively. Hours later, the mice were treated with CO 2 After being anesthetized and sacrificed by cervical dislocation, the tumor tissue was collected, and the total tumor protein was extracted with 200ul of RIPA lysate. The cross-linking detection of PD-L1 was the same as the western blot detection method in Example (2). see results Figure 4 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com