Anti-human CD47 monoclonal antibody
A monoclonal antibody, expression vector technology, applied in the direction of antibodies, anti-animal/human immunoglobulin, anti-tumor drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0062] The acquisition of embodiment 1 hybridoma cell strain
[0063] a. Antigen preparation
[0064] (1) Obtain the target gene
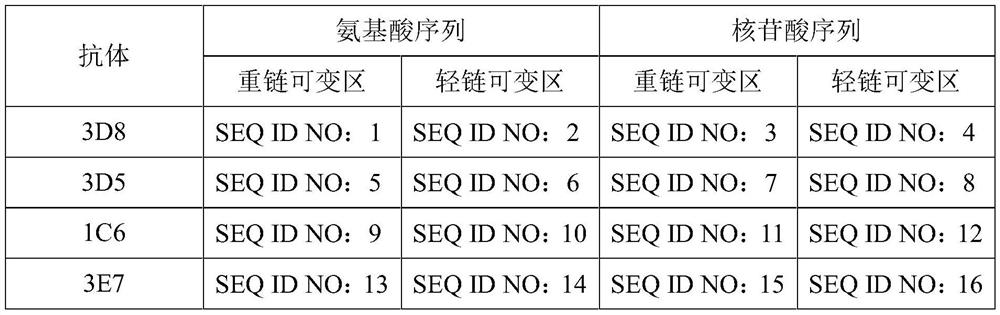
[0065] In this embodiment, the gene sequence of CD47 (NCBI sequence number XP005247966.1, numbered as SEQ ID NO: 25 in the present invention) and its signal is searched from NCBI, and enzyme cutting sites AvrII and BstZ17I are added at both ends, kozak sequence, connecting peptide murine antibody-mFc sequence (NCBI sequence number AAK53870.1, numbered as SEQ ID NO: 26 in the present invention); or adding enzyme cutting sites AvrII, BstZ17I, kozak sequence, -his at both ends Tag, wherein His-tag is a short peptide composed of 6 histidines. The sequence was sent to Shanghai Bioengineering Co., Ltd. for sequence optimization and synthesis. The amino acid sequence design is shown in SEQID NO:17,18. The target gene encoding this protein is synthesized by gene synthesis technology, and its nucleotide sequence is shown in SEQ ID NO:19,20.
[0066] (2)...
Embodiment 2
[0094] Example 2 Preparation and Purification of Antibodies
[0095] The cell lines 3D8, 3D5, 1C6, and 3E7 obtained in Example 1 were recovered, subcultured and expanded, and the cell culture supernatant was collected. Antibodies were purified by Protein A affinity chromatography. First prepare the protein A affinity column. After equilibrating the column with PBS, pass the cell culture supernatant that was centrifuged and filtered through a 0.4um filter through the column, then wash with PBS until the OD value is close to zero, and use 50mmol / L glycine at pH7.5- Elute with hydrochloric acid buffer solution, collect the eluate in the peak area, and set aside after dialysis.
Embodiment 3
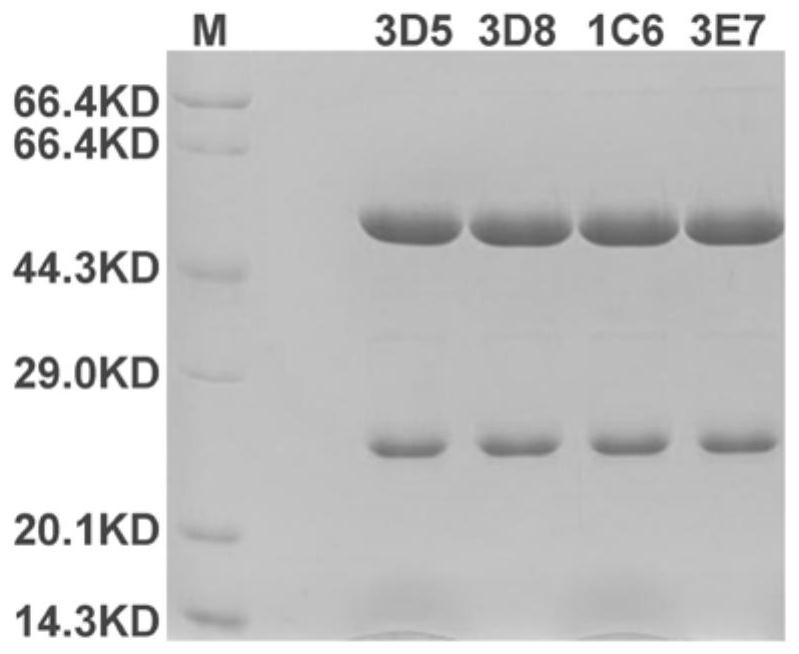
[0096] Example 3 SDS-PAGE detects target protein molecular weight and expression
[0097] The size and purity of the target protein were detected by SDS-PAGE reduction electrophoresis. Electrophoresis was performed according to the method of the fourth part of the Chinese Pharmacopoeia 2015 edition, and the grayscale scanning of the electropherogram was performed to identify the molecular weight and expression level of the monoclonal antibody. According to electrophoresis results figure 1 As shown, the light chain is about 25KD, the heavy chain is about 50KD, and the protein purity is greater than 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com