Method for detecting monoclonal antibody and application thereof
A monoclonal antibody and antibody technology, applied in the field of cell analysis, to reduce the reaction process, accurate and reliable identification results, and reduce interference.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
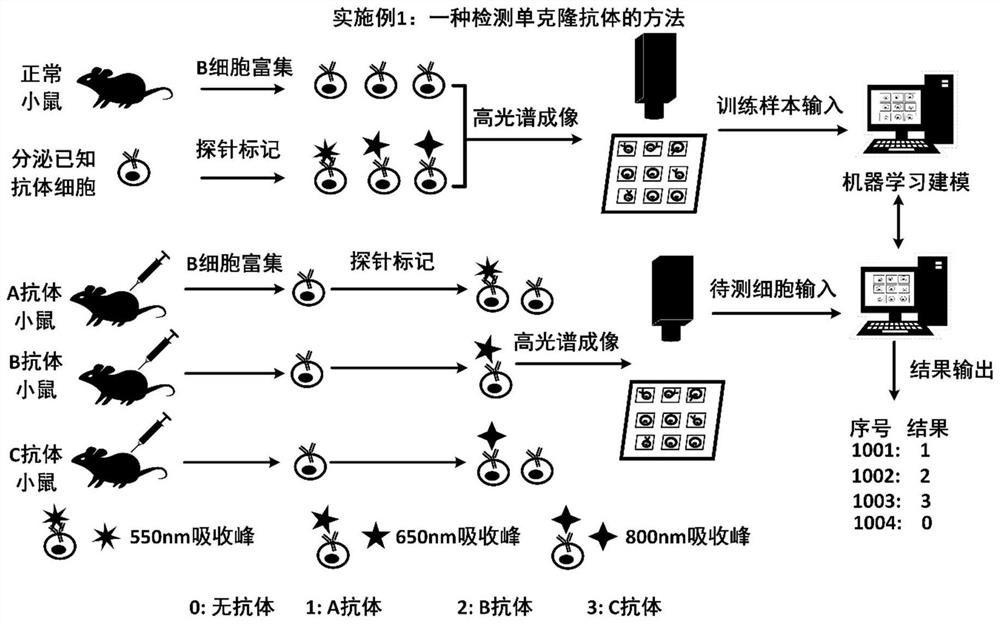
[0059] This embodiment provides a method for detecting monoclonal antibodies, the process is as follows figure 1 As shown, the specific steps include:
[0060] (1) Prepare cells
[0061] Training sample cells: Obtain living cells and / or known cell lines, and prepare single-cell suspensions with buffer. The buffer is 0.85% NaCl physiological saline, phosphate buffer or other buffer solutions suitable for the physiological concentration of the cells. Two processing methods: 1. Add one or more detection antigens and incubate at 4°C, 37°C or room temperature for 1 hour; 2. Prepare single-cell suspension directly without adding antigens. Then wash twice with buffer, and resuspend cells with cell culture medium to prepare a single cell suspension for later use. The cell culture solution is RPMI 1640, DMEM or other nutrient solutions suitable for the growth of the cells.
[0062] Detection of sample cells: Obtain blood, tissues and organs of immunized mice, rabbits and other anim...
Embodiment 2
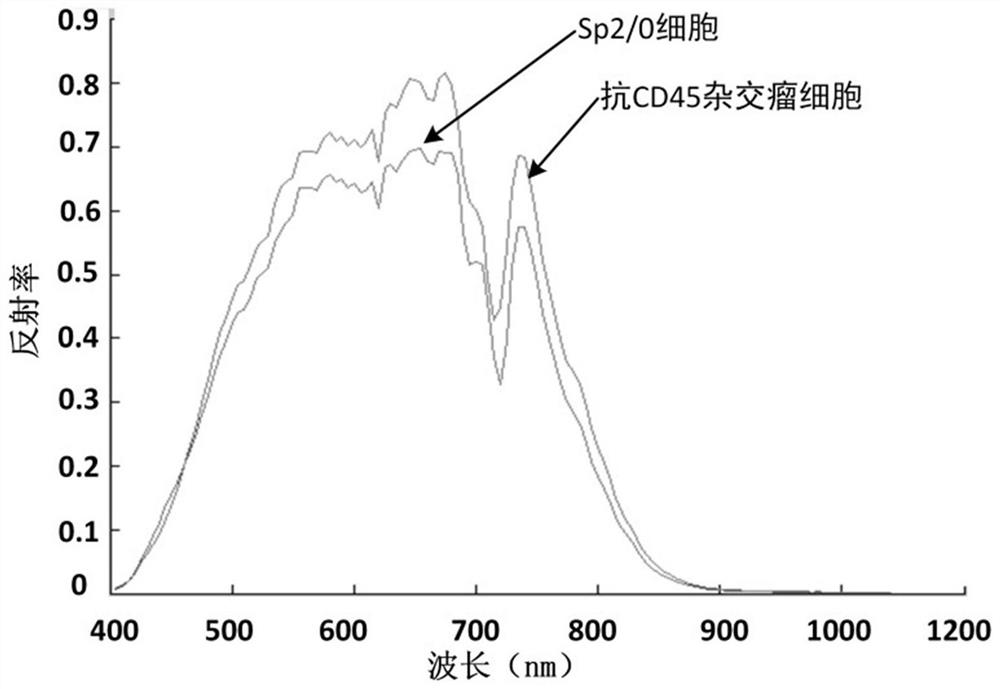
[0100] In this embodiment, using the method in Example 1, the hybridoma cells that secrete anti-CD45 antibody and the Sp2 / 0 cells that do not secrete antibodies are taken as examples, and the specific application methods are described as follows:
[0101] All the experimental steps are carried out in the cell room in a sterile environment, the consumables and instruments are sterilized in advance, and the reagents are also sterile.
[0102] Collect the hybridoma cells and Sp2 / 0 cells, centrifuge at 1000rpm for 3min, resuspend the cells with RPMI1640 culture medium, adjust the cell concentration to 10 per ml 6 cells. 100ul of cell suspensions were taken respectively, added to 96-well plates, and rested for 10 minutes. Spectral information of cells is captured with hyperspectral imaging under a microscope. 10 images were collected for each type of cell. Each type of cell is divided into training set, verification set and inspection set. In the training set, the cells that sec...
Embodiment 3
[0105] In this embodiment, the method of Example 1 is used to screen the cells that secrete PDL1 for the lymphocytes of mice, and the specific application methods are described as follows:
[0106] All the experimental steps are carried out in the cell room in a sterile environment, the consumables and instruments are sterilized in advance, and the reagents are also sterile.
[0107] Take two mice, one is a normal mouse, and the other is a mouse immunized with programmed death protein ligand 1 (PD-L1) protein. The spleen cells of the mice were extracted, ground aseptically, washed twice with serum-free medium, and centrifuged at 1000rpm for 3min to remove fat and other adherents. B cells were isolated from the spleen suspension using mouse B lymphocyte separation medium. Resuspend B cells in RPMI 1640 medium and adjust cells to 10 6 cells. 100ul of cell suspensions were taken respectively, added to 96-well plates, and left to stand for 10 minutes. Cell spectral information...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com