Novel heavy metal absorbent
An adsorbent, heavy metal technology, applied in other chemical processes, chemical instruments and methods, etc., can solve the problems of insufficient active specific surface area, inability to remove mercury, and low adsorption activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
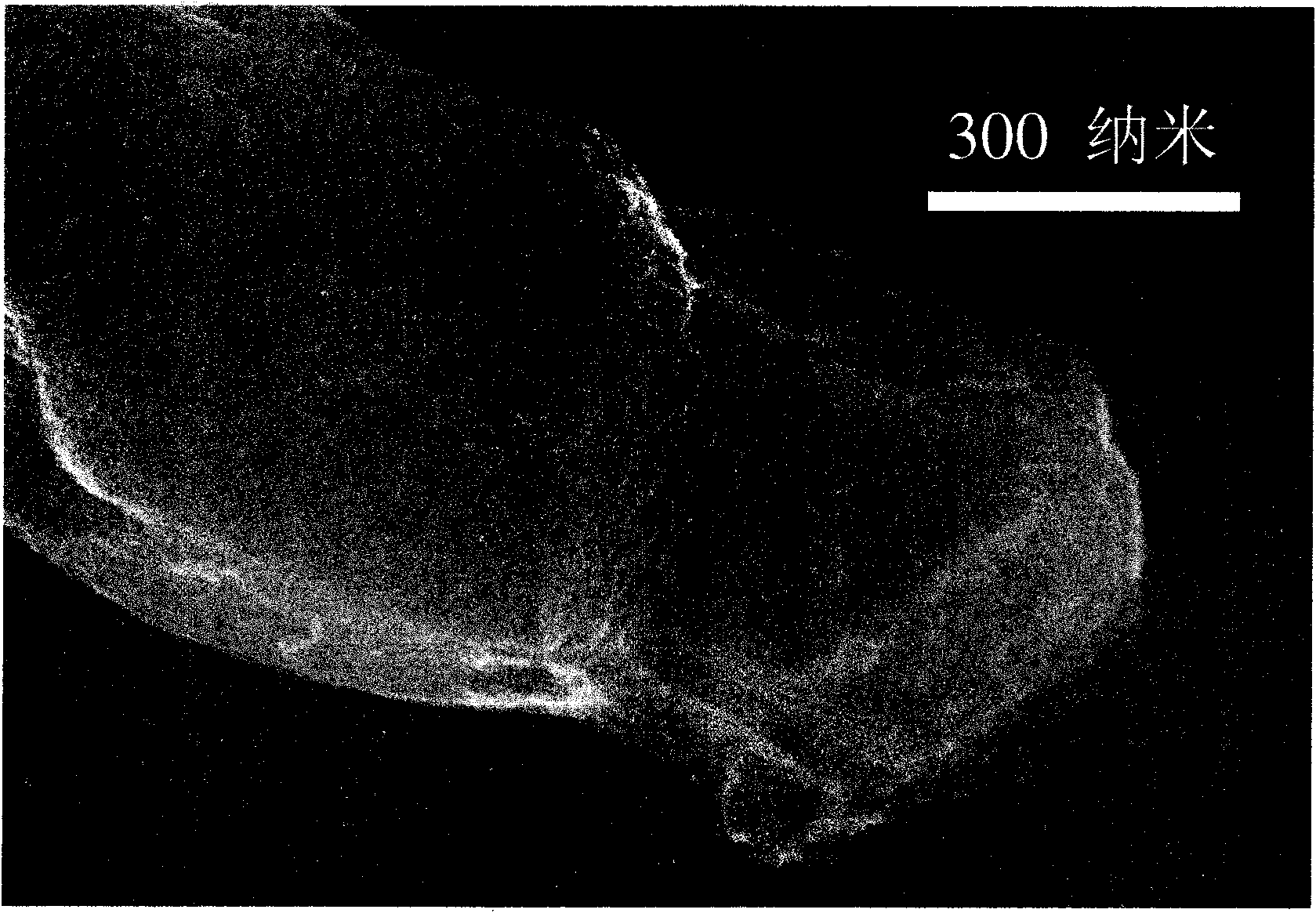
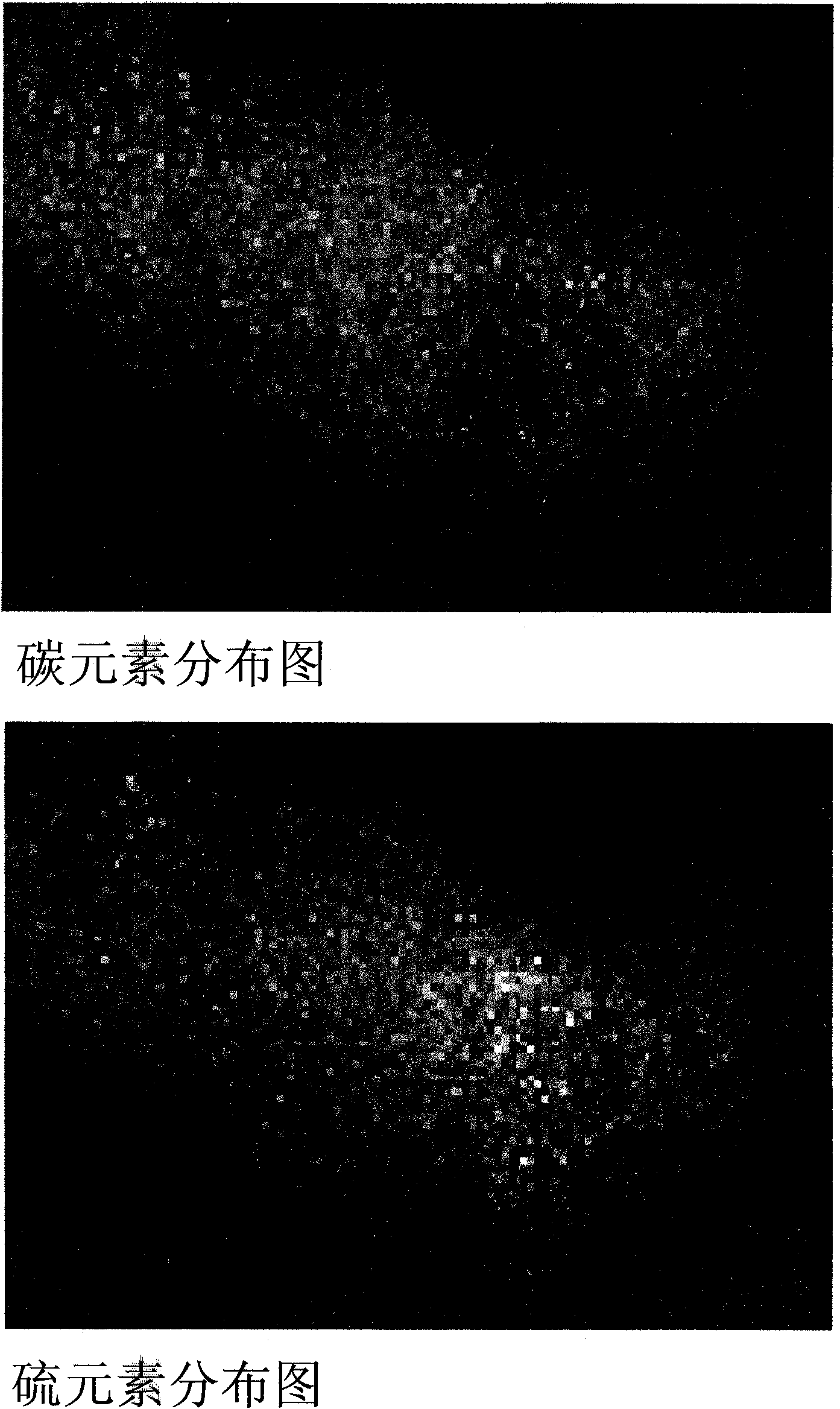
[0018] Disperse 9.5 grams of a mesoporous carbon material (CMK-3) with a regular pore structure and 0.5 grams of commercially available elemental sulfur into a container filled with 500 milliliters of deionized water, and mix the two substances with vortex friction mixing. Mixing was performed by stirring the mixture vigorously for 2 hours using a magnetic stirrer bar. The mixture is filtered to remove water from the mixture. The scanning electron micrograph in Fig. 1 shows the surface morphology of a particle of a sulfur-carbon mixture prepared by the vortex friction mixing method, and a regular strip-like structure can be observed. figure 2 Shown is the X-ray electron spectrogram of the carbon and sulfur elements in the sulfur-carbon mixture particles in Fig. 1. It can be seen that elemental sulfur is evenly distributed throughout the particles of the sulfur-carbon mixture. Combining the information provided by scanning electron microscope photos and X-ray electron spectr...
Embodiment 2
[0020] A kind of commercially available activated carbon, 9.5 grams of graphitized carbon black (Ketjen Black) and 0.5 gram of commercially available elemental sulfur are added to a container filled with 500 milliliters of deionized water, and then the mixture is fully stirred by an electromagnetic stirrer for 2 hours later. The mixture was filtered to remove water from the above mixture. Put the mixture into an oven at 125°C, and after heating for 2 hours, a sulfur-carbon nanocomposite material is prepared.
Embodiment 3
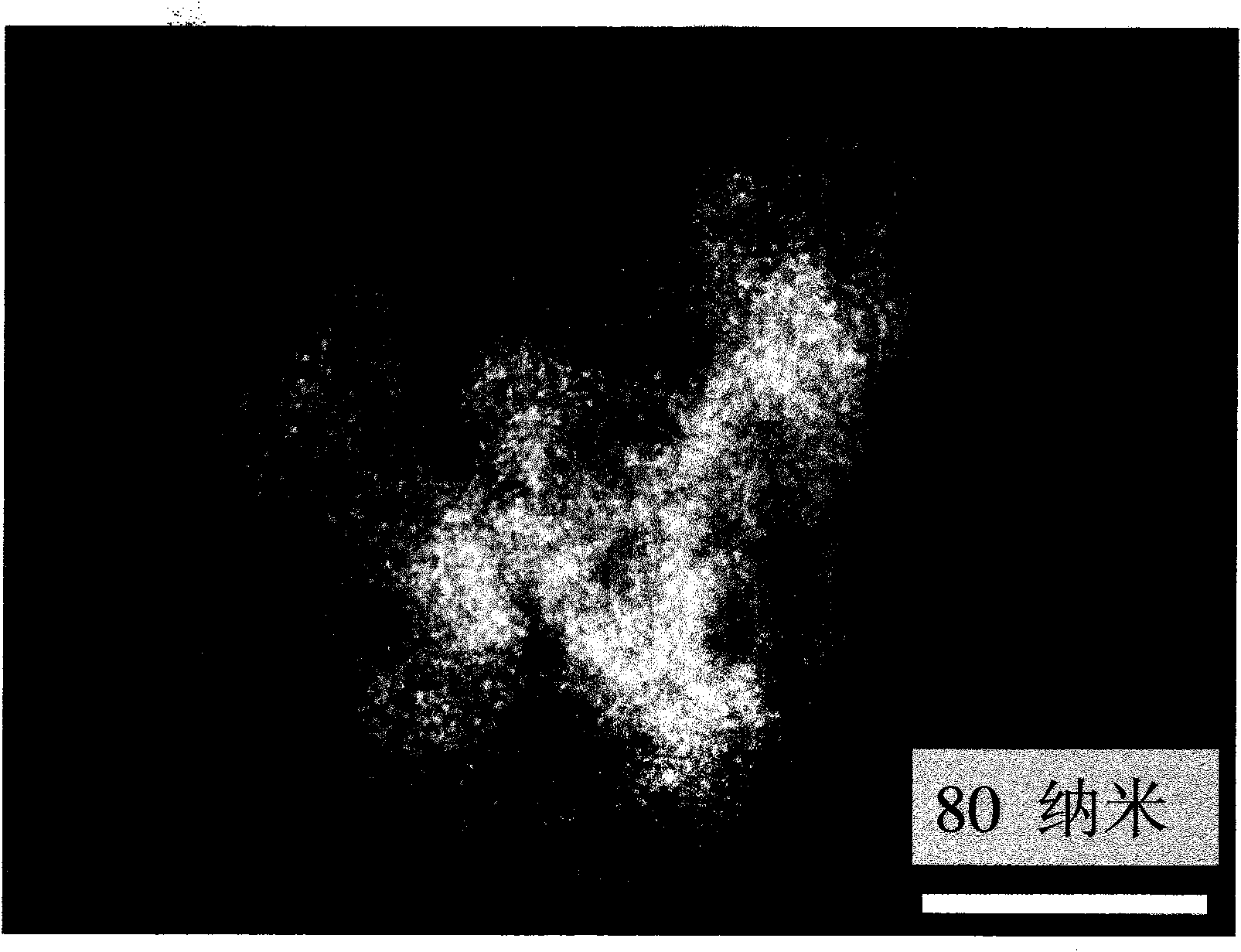
[0022] The sulfur-carbon nanocomposite material 0.1 gram that is synthesized in the embodiment two is dispersed into 2 liters of concentrations and is the chloroplatinic acid (chemical formula is H of 50 parts per million) 2 PtCl 6 ) in an aqueous solution. The mixture was then vigorously stirred for 30 minutes. The solids in the mixture are then filtered to complete the separation of platinum from solution. The black background transmission electron micrograph of the composite material adsorbing perchloroplatinic acid is shown in Figure 3. The bright spot in the distribution in Fig. 3 is from the chloroplatinic acid particles with high electron cloud density adsorbed on the sulfur-carbon nanocomposite. Figure 4 shows the thermogravimetric analysis of the composites adsorbed perchloroplatinic acid. Through the analysis of the results of the thermogravimetric test, it is not difficult to know that the sulfur-carbon nanocomposite material can absorb 35% of its own weight of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com