Ginsenoside-human serum albumin complex injection, preparation method therefor and application of ginsenoside-human serum albumin complex injection
A technology of human serum albumin and ginsenosides, which can be used in pharmaceutical formulations, drug combinations, drug delivery and other directions, can solve problems such as poor water solubility, affecting product yield, denaturation and inactivation, etc., to protect natural conformation and improve killing. effect, effect of strong killing effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0041] The invention provides a preparation method of ginsenoside-human serum albumin complex injection, comprising the following steps:
[0042] 1) mixing human serum albumin with physiological saline to obtain a human serum albumin solution;
[0043] 2) mixing ginsenosides with ethanol-containing physiological saline to obtain a ginsenoside suspension;
[0044] 3) Add the ginsenoside suspension obtained in step 2) dropwise into the human serum albumin solution obtained in step 1), stir, centrifuge to take the supernatant, filter, and take the filtrate;
[0045] The steps 1) and 2) are not limited in chronological order;
[0046] The ginsenosides include ginsenoside Rg5 and / or ginsenoside Rk1.
[0047] In the present invention, the molar ratio of the ginsenoside to human serum albumin is preferably (1:1) to (1:10), more preferably (1:3) to (1:7), most preferably ( 1:4) ~ (1:5). Within the molar ratio range of the present invention, the ginsenoside-human serum albumin comple...
Embodiment 1
[0059] Types and sources of raw materials: ginsenoside Rg5 and ginsenoside Rk1 were purchased from Shanghai Yuanye; human serum albumin was purchased from SigmaAldrich.
[0060] Process flow:
[0061] 1. Weigh 20g of HSA, add it to 100mL of normal saline, stir at 4°C for 3 hours to fully dissolve it (hereinafter referred to as solution A)
[0062] 2. Weigh 100 mg of the mixture of ginsenoside Rg5 and ginsenoside Rk1, add it to 50 mL of physiological saline containing 6% ethanol at room temperature, and stir at room temperature to form a suspension (hereinafter referred to as suspension B).
[0063] 3. At room temperature, add solution A drop by drop to suspension B while stirring, and the dropping speed is controlled at 1mL / min until solution A is completely added to suspension B.
[0064] 4. Move the mixed solution to an environment at 4°C, continue to stir for 3 hours, centrifuge at 20,000×g for 30 minutes, then filter with a 0.22 micron filter membrane, collect the filtere...
Embodiment 2
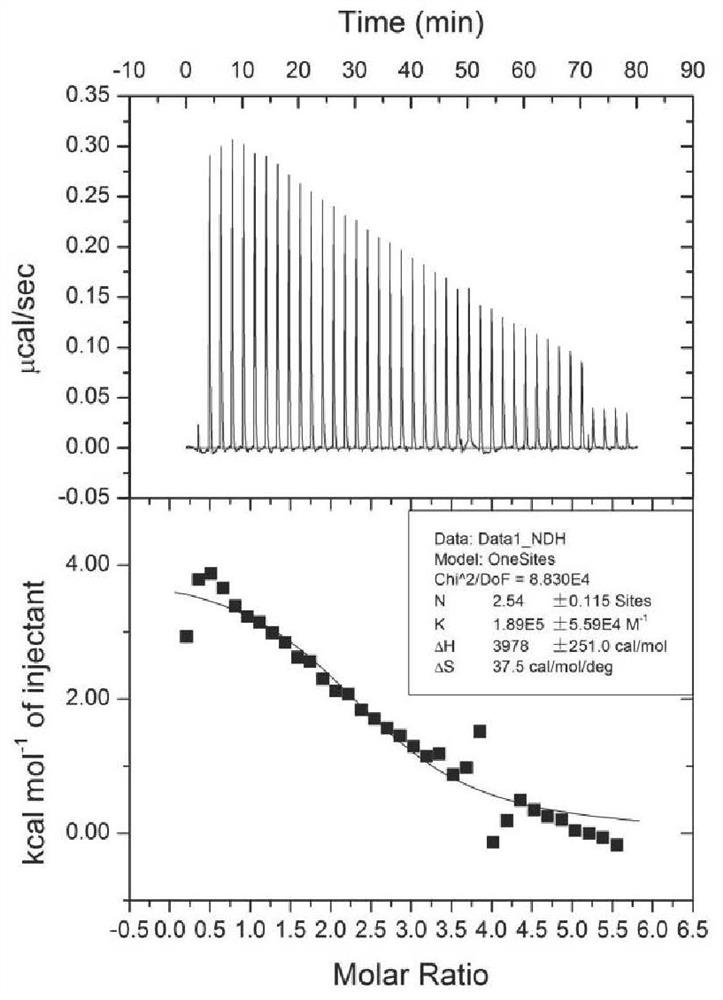
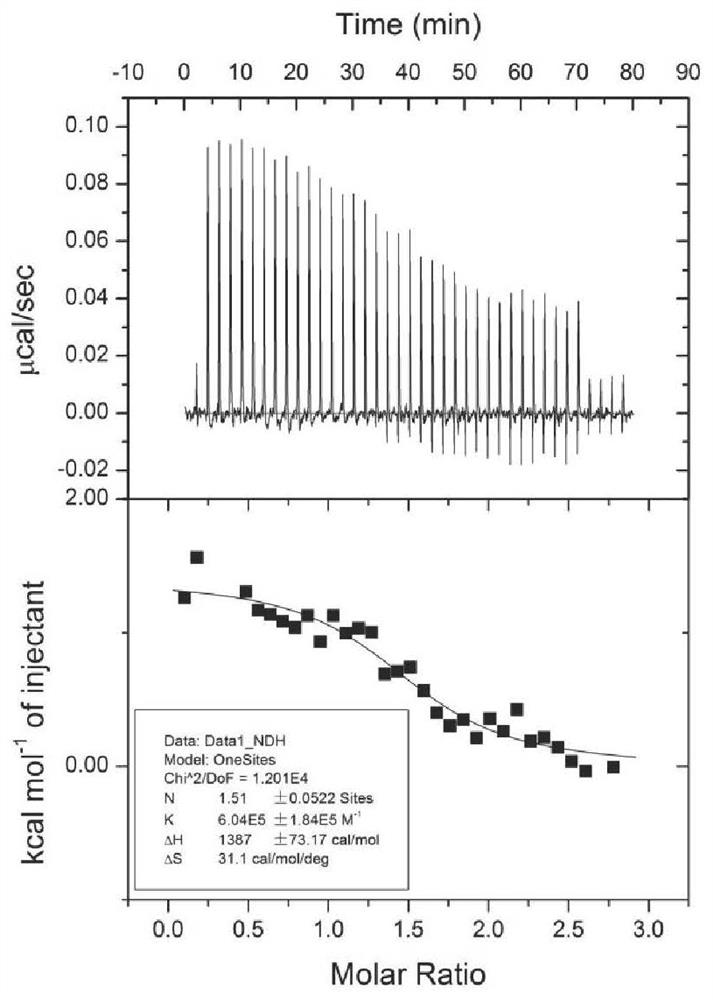
[0066] Determination of Interaction Between Human Serum Albumin and Ginsenosides Rg5 and Rk1 by Isothermal Calorimetric Titration
[0067] Use a Malvern MicroCal ITC200 isothermal calorimeter for measurement, add 200 μL of 20 μM ginsenoside solution (containing 5% ethanol) into the sample pool, draw 40 μL of 400 μM human serum albumin solution (containing 5% ethanol) into the sample needle, and start titration. The result is as figure 1 and figure 2 As shown, among them, figure 1 It is the result of the isothermal calorimetric titration experiment of ginsenoside Rg5 and HSA; figure 2 It is the result of isothermal calorimetric titration experiment of ginsenoside Rk1 and HSA. The abscissa in the figure represents time, the molar ratio of HSA and saponin, and the ordinate represents the heat change during the titration reaction. The results showed that ginsenoside Rg5 and ginsenoside Rk1 could bind to HSA, and the single binding site model was used for fitting, and the bin...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Mass volume concentration | aaaaa | aaaaa |
| Mass volume concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com