mRNA-liposome complex and application thereof
A technology of liposome complexes and liposomes, which can be used in liposome delivery, DNA/RNA vaccination, complete cell/virus/DNA/RNA components, etc., and can solve the problem of high toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0109] Example 1 Preparation of transfection reagent
[0110] In the following examples, the liposome (transfection reagent) InstantFECT comes from PGR-Solutions, Inc.
[0111] InstantFECT contains 1:2w / w lipopolyamine described in formula II and DOPE.
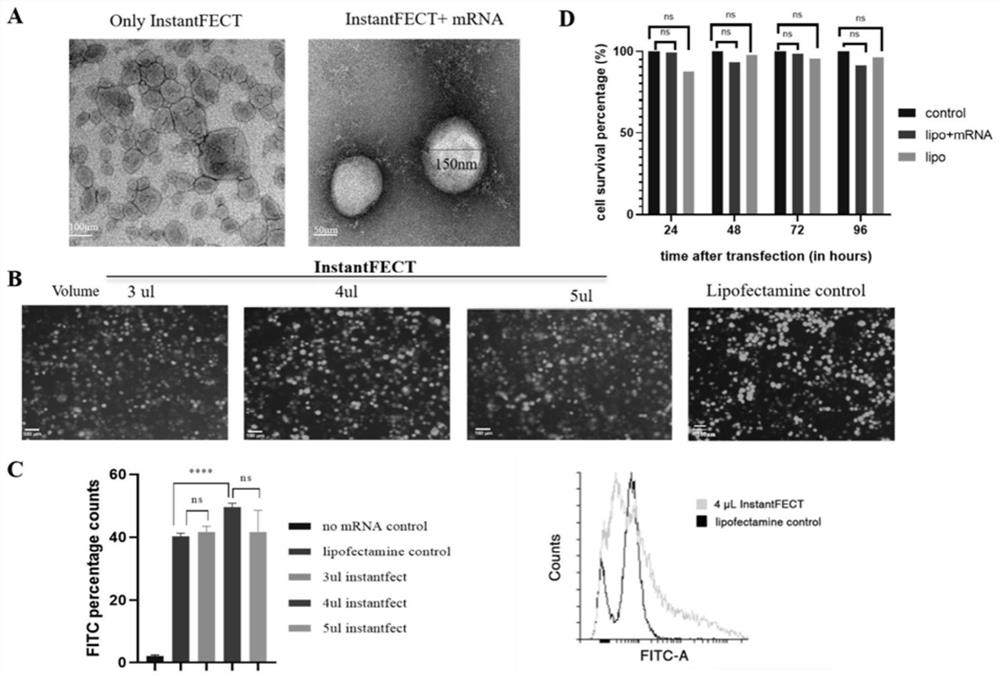
[0112] InstantFECT is a liposome transfection reagent with a unique formula, which can show good transfection efficiency in a relatively wide range of nucleic acid to liposome ratio. At recommended doses, it has very low cytotoxicity and is resistant to serum components during transfection. When using, add the recommended amount (200uL) of the reconstitution solution into the glass bottle, hydrate for one minute, and then vortex for one minute to form a translucent liposome suspension, which is ready to use.
Embodiment 2
[0113] Example 2 mRNA synthesis
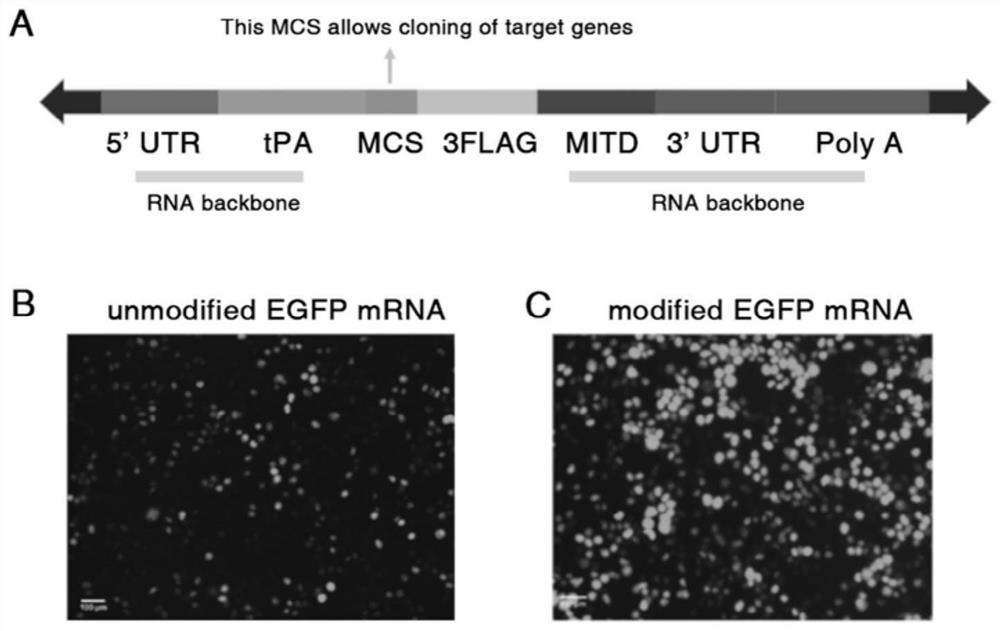
[0114] The 5' and 3' UTR regions of TMV are inserted into the normal pUC57 plasmid, and the poly A tail is inserted at the 3' end to form a DNA backbone. Use NEB's T7Hiscribe mRNA synthesis kit to perform in vitro transcription of plasmid DNA encoded by EGFP (green fluorescent protein), ADSa (Staphylococcus aureus adenosine synthase A) and OVA (ovalbumin) (see the kit instructions for specific steps) . A mixture of DNA backbone (containing gene, TMV 5'UTR, TMV 3'UTR, and poly A tail), NTPS (ATP, CTP, GTP, UTP, and pseudouridine), T7 polymerase, and buffer was incubated at 37 °C Incubate for 2-3 hours and purify the mRNA product by LiCl precipitation. The modified in vitro transcribed mRNA was then capped using the NEBCenovia viral capping enzyme cap1 system. After purification by Licl precipitation, the 5'cap-modified mRNA products were stored at -20°C.
[0115] We chose the portion encoding amino acid 241-amino acid 339 in the ovalbumin g...
Embodiment 3
[0120] The preparation of embodiment 3 mRNA liposome complexes
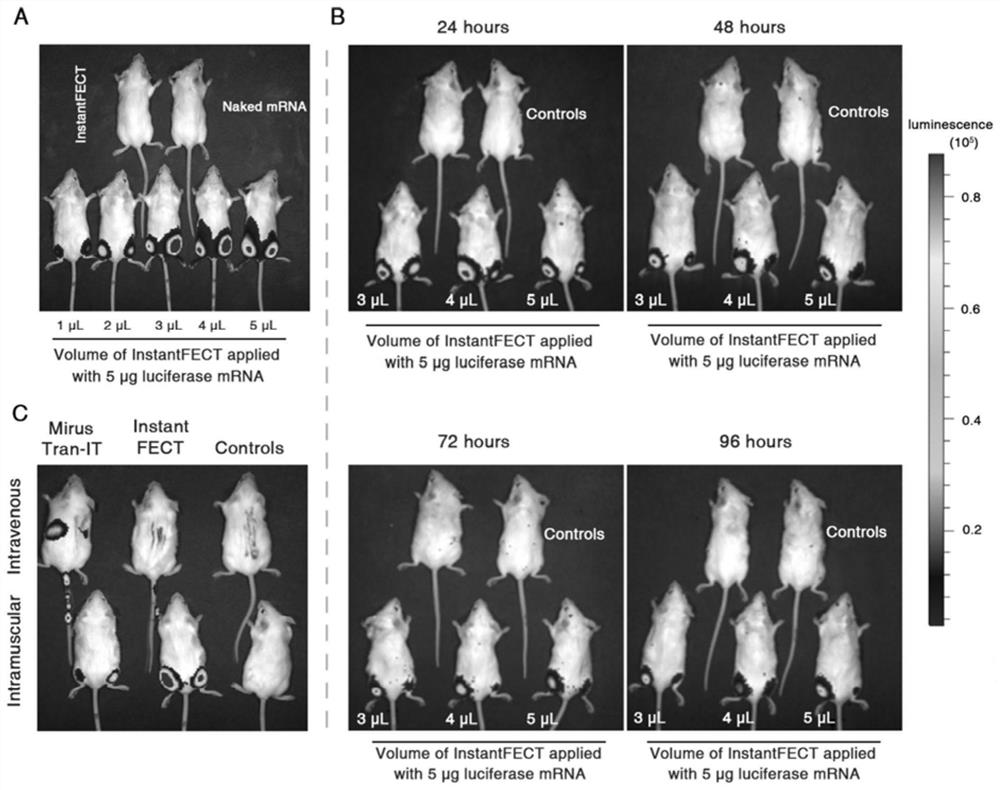
[0121] The method of mRNA lipid nanocomplex was as follows: 5 μg mRNA was diluted with 0.9% NaCl, mixed with 4 μl of InstantFECT and used within 15 minutes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com