Method for forming hydrophobic electrically conductive microporous layer for use as gas-diffusion layer
A gas diffusion layer, microporous layer technology, used in climate sustainability, electrical circuits, fuel cells, etc., to solve problems such as surface inhomogeneity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Synthesis of Poly(2,3,4,5,6-Pentafluorostyrene)(PPFS)
[0088] In a round bottom flask, 500 μl of TiCl 4 Added to 6ml of toluene. The catalyst was prepared by adding dropwise thereto 750 μl of a solution of triethylaluminum in 6 ml of toluene. The mixture was allowed to stabilize for 30 minutes.
[0089] Subsequently, 1.5 ml of TiCl will be formed 4 / AlEt 3 The catalyst system was added all at once to 3 ml of 2,3,4,5,6-pentafluorostyrene (PFS) sold by Sigma-Aldrich under the number 196916-25G (CAS: 653-34-9).
[0090] The mixture was stirred and kept at 70°C for 12 hours, then at 120°C for 36 hours.
[0091] The obtained product was dissolved in fluorobenzene, and then the catalyst was neutralized with ethanol, followed by filtration.
[0092] The polymer solution was precipitated from methanol.
[0093] A mass yield of 92% was obtained.
[0094] The number average molecular weight determined by size exclusion chromatography (SEC) with triple detection was M n ...
Embodiment 2
[0099] Preparation of inventive or non-inventive microporous layers
[0100] Dispersions of carbon particles according to the invention and PPFS in NMP (Test 1), and non-inventive dispersions in water with polytetrafluoroethylene (PTFE) instead of PPFS (Comparative Test 2) were prepared as follows:
[0101] carbon black (Vulcan ) and carbon fibers produced in the gas phase mix. The hydrophobic polymer in question is then introduced, followed by the solvent (NMP or water). The mixture was then dispersed in a Dispermat under vacuum (-0.9 bar) at 6000 rpm for 20 minutes to form a dispersion.
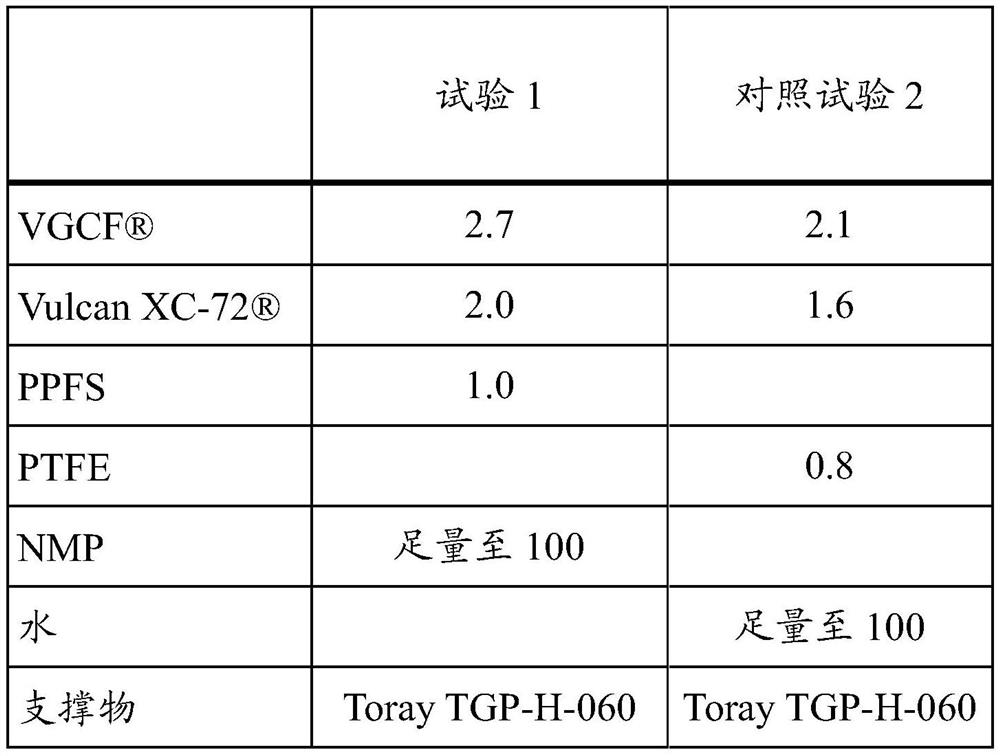
[0102] Table 1 below lists the proportions (expressed in mass percent relative to the total mass of the dispersion) used for each dispersion tested and the supports used.
[0103] [Table 1]
[0104]
[0105] The dispersions were deposited on Toray TGP-H-060 supports for Trial 1 and Comparative Trial 2 at ambient temperature using a knife coating bar with a height of 200 μm.
[01...
Embodiment 3
[0110] Characterization of the microporous layer obtained in Example 2
[0111] The surface properties of the microporous layer obtained in Example 2 were evaluated by water droplet deposition. All deposits obtained from the various tests showed a hydrophobic surface. It should be noted that, on the contrary, this property was obtained for Control Experiment 2 only after heat treatment.
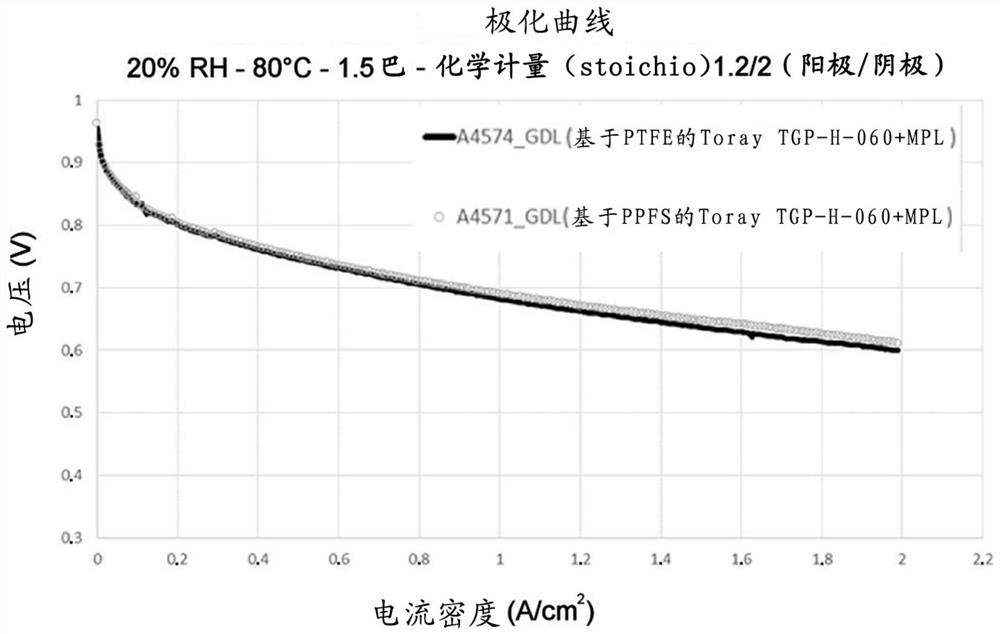
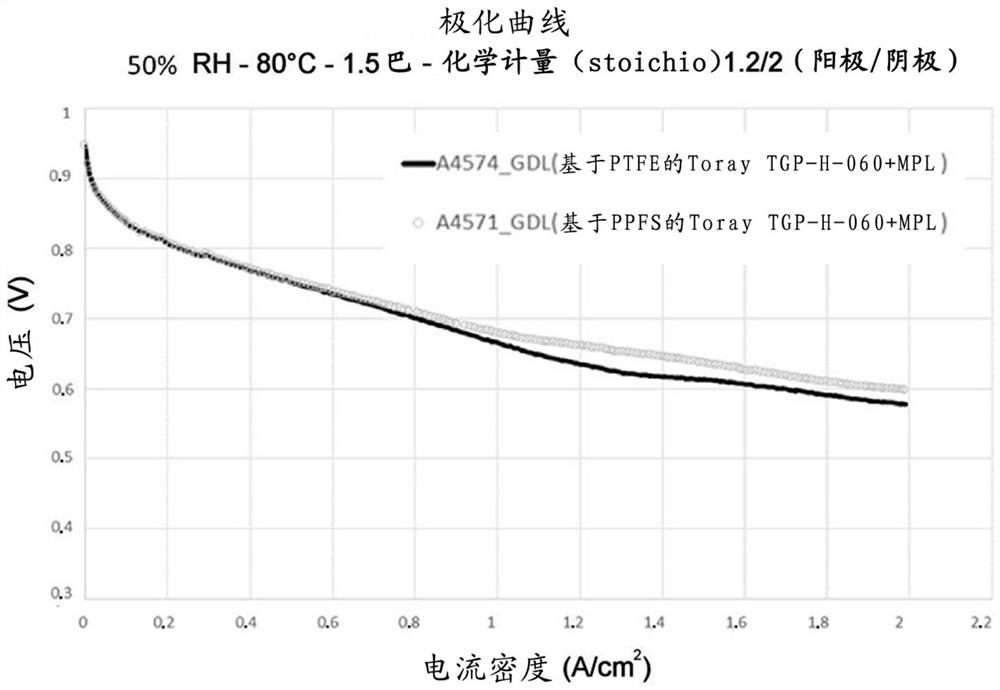
[0112] These various microporous layers formed on carbon supports were used as gas diffusion layers in fuel cells, and the electrochemical performance of the gas diffusion layers was also tested according to the protocol described above in the "Equipment and Technique" section.
[0113] figure 1 and figure 2 The polarization curves measured at 20% and 50% relative humidity for the microporous layer obtained in Example 2 for Test 1 and Control Test 2 are shown, respectively. These two microporous layers on Toray TGP H 060 supports performed similarly, reaching 2A / cm at a voltage of about ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com